Nucleoside Triphosphate Derivatives of Favipiravir Analogues as Potent and Broadly Acting Antivirals
The nucleobase analogue favipiravir (T-705) is a promising starting point for the development of broadly acting antivirals as it has demonstrated activity against numerous RNA viruses. However, the nucleoside form of this analogue revealed to be chemically unstable (Huchting et al. ChemMedChem 2017 ).
T-1105, the non-fluorinated version of T-705, has favorable chemical stability and greater antiviral potency in Madin-Darby Canine Kidney (MDCK) cells (Huchting et al. ChemMedChem 2017; Huchting et al. J. Med. Chem. 2018; Huchting et al. Antiviral Res. 2019). Yet, in other models this analogue proved less potent due to metabolic bottlenecks in the activation pathway Nb* -> N*MP -> N*DP -> N*TP. These depend on the chemical structure of Nb* and, importantly, on the cell type which is used for studying antiviral activity (Huchting et al. Antiviral Res. 2019).
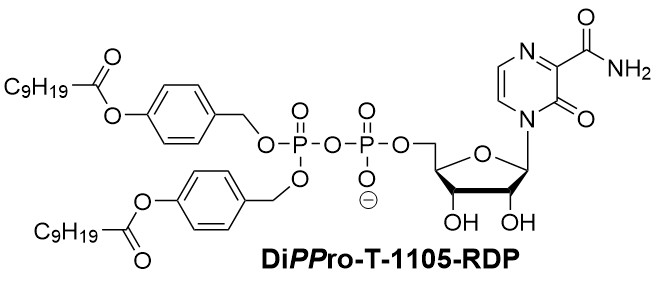
We therefore apply our DiPPro- and TriPPPro-technology to bypass inefficient host cell kinases and ultimately deliver high levels of N*TP to their viral target within infected cells. For example, DiPPro-T-1105-RDP inhibited influenza virus with 5-fold greater potency than the nucleobase analogue T-1105 itself in cell culture (Huchting et al. J. Med. Chem. 2018). Further, we optimize chemical synthesis of N*TP to study their properties towards different viral RNA polymerases (Huchting et al. J. Med. Chem. 2018) and to ultimately find novel nucleoside analogue triphosphates with favorable characteristics as candidates for broadly acting therapeutics against RNA virus infections.