Prof. Dr. Bernd Meyer
Im Ruhestand
AK Meyer
Anschrift
Kontakt
Schwerpunkte
- Analysis of Glycoconjugates by Nuclear Magnetic Resonance (NMR) and Mass Sprctrometry (MS)
- Analysis of Ligand-Receptor Interactions by Saturation Transfer Difference NMR (STD NMR)
- and by Surface Plasmon Resonance (SPR)
- Synthesis of N-linked Glycopeptides
- Desigin and Synthesis of Inhibitors of Glycosyl-Transferases
- Desigin and Synthesis of Antiviral Compounds
Curriculum Vitae
| Date of Birth: | 16th March 1952 |
| Ph.D. thesis: | 1979, University of Hamburg, title of the dissertation: "Structural Definition of the cytostatica chromo-mycine A, mithramycin and livomycin A. Synthesis of their olivomosyloliodide as well as their isomeric disaccharides.” |
| Post Doctorate: | 1979 to 1980, Technical University of Denmark, Lyngby, Prof. Dr. K. Bock, Studies of Glucoseisomerase and First Modeling Studies of Carbohydrates |
| Scientific Associate | 1980-1986, University of Oldenburg |
| Habilitation | 1986, Habilitation in Organic Chemistry, subject: “Chemistry at the University of Oldenburg. Modelling of Oligosaccharides and Glycoconjugates” |
| Privatdozent | 1986 - 1988, University of Oldenburg |
| Assistant Professor | 1988 - 1992, Dept. of Biochemistry and Complex Carbohydrate Research Centre, University of Georgia, Athens, Ga., USA |
| Associate Professor and Tenure | 1992 - 1993, Dept. of Biochemistry and Dept. of Chemistry of the University of Georgia, Complex Carbohydrate Research Centre, Athens, Georgia, USA |
| Professor | since 1993, Organic Chemistry at the University of Hamburg |
Awards
- Belfort Lecturer, Purdue University, 1996
Organization
-
Organizer und Co-Organizer of several international conferences
-
Vize speaker of the SFB 470, 1997-2006: Glycostructures in biological systems
-
Speaker of the graduate program, 1998-2004: Glycostructures: Synthesis, Analysis, Structure and Function
Collaborations
-
Prof. Dr. med. Christoph Wagener, University Medical Center Hamburg-Eppendorf, Hamburg
-
Prof. Dr. Christian Drosten, University of Bonn Medical Center, Institute for Virology, Bonn
-
Prof. Dr. Thomas Peters, University of Luebeck, Institute of Chemistry, Luebeck
-
Prof. Dr. Eckhard Mandelkow und Dr. Eva Mandelkow, Max Planck Institute for Structural Molecular Biology, Hamburg
-
Prof. Dr. Dr. Christian Betzel, University of Hamburg, Institute of Biochemistry and Molecular Biology, Hamburg
-
Prof. Dr. Reinhard Bredehorst, University of Hamburg, Institute of Biochemistry and Molecular Biology, Hamburg
- PD Dr. Edzard Spillner, University of Hamburg, Institute of Biochemistry and Molecular Biology, Hamburg
- Bruker BioSpin GmbH, Rheinstetten
Publikationen
Research Focus
NMR Analysis of Glycoconjugates
We analyze by high field NMR spectroscopy the structure of oligosaccharides that are present in glycoconjugates. This includes the primary structure and the three dimensional structure. Mainly, NMR spectroscopy and mass spectronomy is being used for these types of analyses. It is paramount to characterize even minor amounts of oligosaccharides on glycoproteins to understand their function in the biological system. Presence of immunogenic or structures that influence the clearance of glycoproteins from the body is very important. Also, the structure of oligosaccharides, which influence uptake of glycoproteins or that affect cell-cell interactions, is important for the function of glycoproteins.
Characterization of picomole amounts of oligosaccharides from glycoproteins by 1H NMR spectroscopy.
Fellenberg, M.; Coksezen, A.; Meyer, B.,
Angew Chem Int Ed Engl 2010, 49, (14), 2630-3.
Identification of the 1H-NMR Spectra of Complex Oligosaccharides with Artificial Neural Networks.
B. Meyer, T. Hansen, D. Nute, P. Albersheim, A. Darvill, W. York & J. Sellers,
Science 251, 542-544 (1991).
Sensitivity Enhancement by Artificial Neural Network Based Recognition of 1H-NMR Spectra. Application to N-Type Oligosaccharides from Glycoproteins.
J.P. Radomski, H. van Halbeek & B. Meyer,
Nature Struct. Biol. 1, 217-218 (1994).
The V3-directed immune response in natural human immunodeficiency virus type 1 infection is predominantly directed against a variable, discontinuous epitope presented by the gp120 V3 domain.
M. Schreiber, C. Wachsmuth, H. Müller, S. Odemuyiwa, H. Schmitz, S. Meyer, B. Meyer, J. Schneider-Mergener,
J. Virol. 71, 9198-9205 (1997).
Stabilization of the Peptide Backbone through Interactions with 12 O-Glycans and one N-Glycan: NMR study of the T1 Fragment of Glycophorin AN,
J. Pieper, K.H. Ott & B. Meyer,
Nature Struct. Biol. 3, 228-232 (1996).
‘Wave-type’ structure of a synthetic hexaglycosylated decapeptide: A part of the extracellular domain of human glycophorin A.
Oliver Schuster, Gunther Klich, Volker Sinnwell, Helge Kränz, Hans Paulsen & Bernd Meyer,
J. Biomolecular NMR, 14, 33–45, (1999).
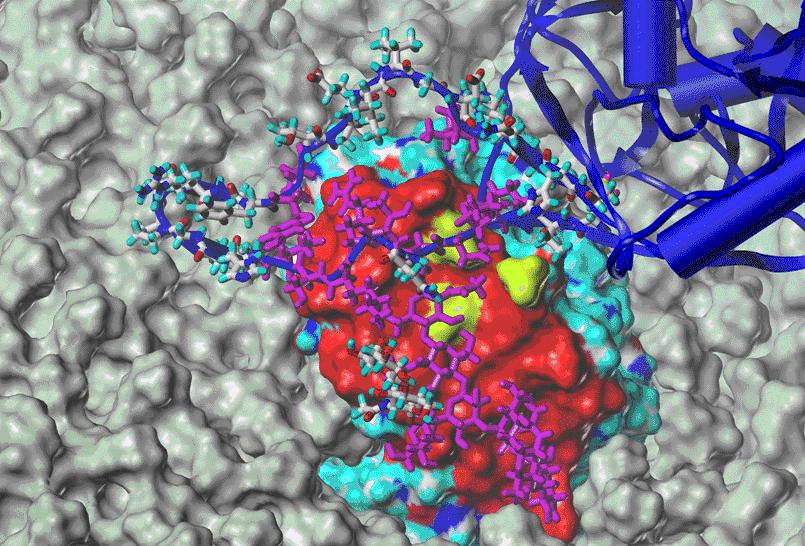
Model of the binding of the viral GP120 of HIV to the human coreceptor CCR5 embedded in a membrane using NMR and surface plasmon resonance studies; blue: GP120 - the V3 loop of GP120 is shown as a stick plot; red: CCR5 binding epitope; by atom color: amino acids from the CCR5 as a surface plot.
STD NMR Analysis of Ligand-Receptor Interactions
Saturation Transfer Difference NMR spectroscopy (STD NMR)
We have developed the STD NMR protocol to elucidate ligand-receptor interactions by NMR. We can characterize the binding epitope of the ligands to the receptor and can achieve a very high sensitivity in terms of amount of protein required. We can also determine KD values and association and dissociation kinetics (koff and kon).The receptor can be any type of protein either being soluble or even membrane integrated. Even proteins on the surface of native whole cells can be analyzed. At the minimum, the need for protein is down to about 30 picomols of protein to obtain an STD NMR spectrum.
Direct observation of ligand binding to membrane proteins in living cells by STDD NMR,
Birgit Claasen, Marko Axmann, Robert Meinecke & Bernd Meyer,
J. Am. Chem. Soc. 127, 916-919 (2005).
Group Epitope Mapping (GEM) by STD NMR to Identify Segments of a Ligand in Direct Contact with a Protein Receptor.
Moriz Mayer and Bernd Meyer,
J. Am. Chem. Soc. 123, 6108-6117 (2001).
Detecting Binding Affinity to Immobilized Receptor Proteins in Compound Libraries by HR-MAS STD NMR,
Jens Klein, Robert Meinecke, Moriz Mayer & Bernd Meyer,
J. Am. Chem. Soc. 121, 5336-5337 (1999).
A Fast and Sensitive Method to Characterize Ligand Binding by Saturation Transfer Difference NMR Spectra,
M. Mayer & B. Meyer,
Angew. Chem. Int. Ed. 38, 1784-1788 (1999); Angew. Chem. 111, 1902-1906 (1999).
Analysis of Specificity and Epitope of Ganglioside Oligosaccharides Binding to Myelin Associated Glycoprotein (MAG) by Saturation Transfer Difference NMR,
So-Young Shin, Heiko Gäthje, Oliver Schwardt, Beat Ernst, Soerge Kelm and Bernd Meyer,
ChemBioChem 9, 2946 – 2949 (2008).
Example of the utility of a saturation transfer double difference to identify binding receptors in living cells. Double difference saturation transfer is necessary if cells with active metabolism are being analyzed as a target carrying the receptor under investigation. The top trace shows the STD NMR spectrum of platelets with added cyclic peptide, the middle trace shows a different sample prepared under identical conditions containing only platelets. The difference of the top and the middle trace yields a clean saturation transfer double difference NMR (STDD NMR) spectrum that gives only information about the binding of the cyclic peptide plus some residual signals from buffer molecules.
SPR Analysis of Ligand-Receptor Interactions
Surface Plasmon Resonance (SPR)
We use surface plasmon resonance (Biacore T100 and Biacore J) to analyze binding properties of ligands to receptor molecules. Here, the receptor molecules are immobilized on the surface with various immobilization protocols. Interactions between ligands and proteins can be measured by the surface plasmon resonance analysis to determine the binding affinity. Binding kinetics can often also be determined. Distinction between specific and non-specific binding is easily possible.
Phenylthiazolyl-Hydrazide and Its Derivatives Are Potent Inhibitors of τ Aggregation and Toxicity in Vitro and in Cells,
Marcus Pickhardt, Gregor Larbig, Inna Khlistunova, Atilla Coksezen, Bernd Meyer, Eva-Maria Mandelkow, Boris Schmidt, and Eckhard Mandelkow,
Biochemistry; 46, 10016 – 10023, (2007).
Discovery and Optimization of a Natural HIV-1 Entry Inhibitor Targeting the gp41 Fusion Peptide
Jan Münch, Ludger Ständker, Knut Adermann, Axel Schulz, Michael Schindler, Raghavan Chinnadurai, Stefan Pöhlmann, Chawaree Chaipan, Thorsten Biet, Thomas Peters, Bernd Meyer, Dennis Wilhelm, Hong Lu, Weiguo Jing, Shibo Jiang, Wolf-Georg Forssmann, and Frank Kirchhoff,
Cell 129, 263–275, ( 2007).
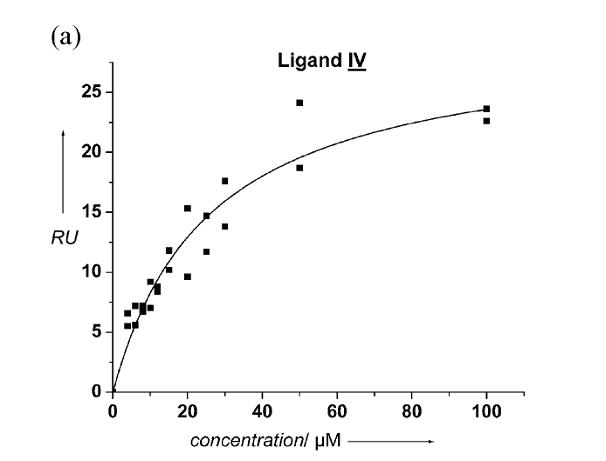
Binding curve of a peptidomimetic ligand to CD4 that was developed by optimization of the binding affinity (cf. inhibitor design section). Shown is ligand binding in a surface plasmon resonance assay performed on Biacore T100 instrument for a ligand to CD4 is clearly visible that the increase of resonance units plotted on the Y access as a function of the concentration in micro molar is representing a saturable binding of that ligand to the CD4 receptor protein of HIV.
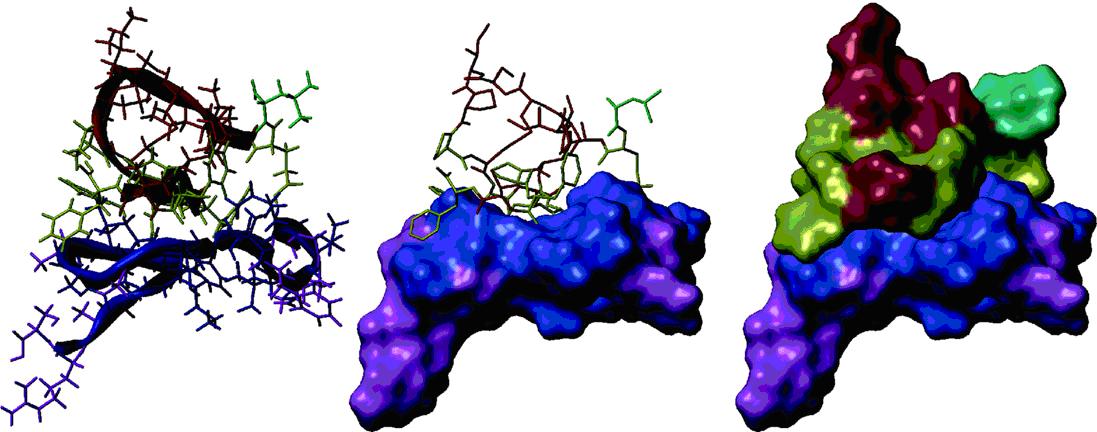
A model derived by NMR spectroscopy and surface plasmon resonance for the interaction of an α1 chymotrypsin peptide directed against a section of GP41 of HIV. left: Stick model plus ribbon of the two peptides; middle: one peptide shown as a surface plot; right: both peptides shown as a surface plot.
Glycopeptide Synthesis
To analyze the interaction between glycopeptides and receptor proteins, we synthesize peptides and glycopeptides to elucidate the function of the carbohydrates on the receptor-ligand interaction. Here, the glycopeptides mimic sections of the glycoproteins that cannot easily be handled as a ligand. We found a number of interactions where a mixed peptide-carbohydrate epitope is responsible for the binding process. We utilize microwave assisted peptide and glycopeptide synthesis on the solid phase as a routine protocol. These projects are aimed at identifying and characterizing protein-protein interactions that are involving carbohydrate components in the recognition by the receptor protein.
Epitope Mapping and Conformational Analysis of MUC-1 Glycopeptides and Peptides Bound to the Breast Cancer-selective Monoclonal Antibody SM3. Heiko Möller, Nida Serttas, Hans Paulsen, Joyce Taylor-Papadimitriou, and Bernd Meyer,
Eur. J. Biochem. 269,1444-1455, (2002).
Synthesis and characterization of highly glycosylated glycopeptides with Tn antigenic structures of the human glycophorin AN. G. Klich, H. Paulsen, B. Meyer, M. Meldal, K. Bock,
Carbohydr. Res. 299, 33-48 (1997).
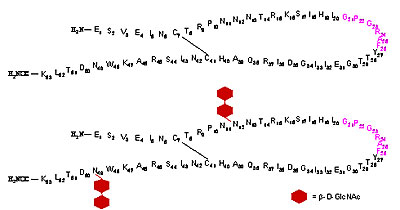
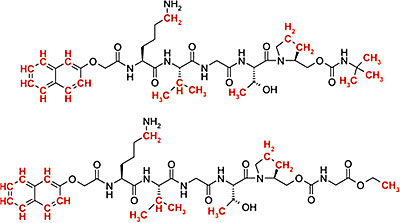
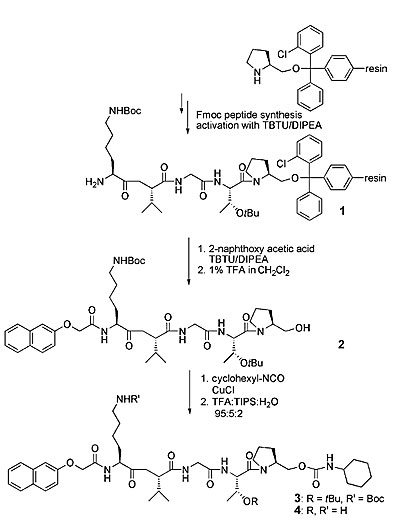
Example for the efficiency of glycopeptide synthesis. Here, 53mer peptide and glycopeptide of the V3 loop of GP120 are shown. The bottom molecule represents a glycopeptide with two N-type glycosylation sites with attached chitobiose.
Design and Synthesis of Inhibitors of Enzymes and Biomolecular Interactions
Starting from peptides that are often identified from protein-protein interactions as binding entities, we characterize these weekly binding peptides and their binding epitope by STD NMR and then modify the affinity of these peptides by elaborate synthesis and computer modeling cycles. Repeated refinement of these structures can lead to an improvement of binding constants by a factor of more than 10,000. We have obtained very successfully compounds that block the CD4 receptor on human cells and are continuing the project towards more generalized enzyme-ligand interactions.
Rational optimization of the binding affinity of CD4 targeting peptidomimetics with potential anti HIV activity. Neffe AT, Bilang M, Grüneberg I, Meyer B.,
J. Med. Chem. 50, 3482-8, (2007).
Synthesis and optimization of peptidomimetics as HIV entry inhibitors against the receptor protein CD4 using STD NMR and ligand docking. Neffe AT, Bilang M, Meyer B., Org Biomol Chem. 4, 3259-67, (2006).
Synthesis of Neu5Ac oligosaccharides and analogues by transglycosylation and their binding properties as ligands to MAG. Neubacher B, Scheid S, Kelm S, Frasch AC, Meyer B, Thiem J.
ChemBioBhem. 7, 896-9 (2006).
A New Peptidomimetic HIV Entry Inhibitor Directed Against the CD4 Binding Site for the Viral GP120, Axel T. Neffe & Bernd Meyer,
Angew. Chem.116, 2997 – 3000 (2004).
Two molecules designed by repeated modeling, STD NMR analysis and SPR analysis that bind selectively to the binding site of GP120 on CD4.
Synthesis path to obtain mimetics for the interaction of GP120 with CD4 with low micromolar binding affinities.
Patents
-
Jan Thomsen, Jeffery Sellers and Bernd Meyer, Apparatus and Methods for Identification of Materials Using Neural Networks, U.S. Pat. No. US5218529
-
T. Peters, B. Meyer, Verfahren zum Nachweis biologisch aktiver Substanzen in Substanzbibliotheken. Ger. Pat. No. DE19649359.
-
T. Peters, B. Meyer, Verfahren zum Nachweis biologisch aktiver Substanzen in Substanzbibliotheken. Swiss Pat. No. 690695.
-
T. Peters, B. Meyer, Method for detecting biologically active compounds from compound libraries, U.K. Pat. No. GB23211401.
-
T. Peters, B. Meyer, Method for detecting biologically active compounds from compound libraries, U.S. Pat. No. US6214561.
-
Schreiber, Michael, Meyer, Bernd, Seifert, Annette. Identifying compounds that modify interaction of gp120 and co-receptors, useful potentially for treating human immune deficiency virus infection, also new peptides. Ger. Pat. No. DE10113042.
-
Scheid, Svenja, Meyer, Bernd, Kelm, Sorge, Inhibitors of the myelin associated glycoprotein, WO 2009/036754 A1
Equipment
Owned by the groupNMR Spectrometers
Surface Plasmon Resonance
Mass Spectrometry
Liberty Microwave Peptide SynthesizerHP Workstations xw 6400 for Molecular Modelling and Design (SYBYL 8)We also use centrally located equipment:Surface Plasmon Resonance
Mass Spectrometry
|