Prof. Dr. Joachim Thiem
Im Ruhestand
AK Thiem
Anschrift
Kontakt
Curriculum Vitae
- Chemistry study and Dissertation in Hamburg with Prof. Dr. H. Paulsen (1972)
- Habilitation (1978) and Assistant Prof.in Hamburg (till 1982)
- Associate Prof. in Münster (1983-1989)
- Full Prof. in Hamburg (since 1989)
- Sabbaticals and Visiting Professorships: University of California, Berkeley, CA, USA (1984/85)
- Victoria University and IRL, Wellington, NZ (1993)
- CERMAV, Grenoble, F (1995)
- The Scripps Research Institute (TSIR), La Jolla, CA, USA (2005/06).
Public Service
- Member of Scientific Workgroup of Industrial Research Committee (1984-1992)
- Member of Executive Board FEI (1989-2007)
- Confidential Docent of FCI (1991-2010)
- German Representative for European Carbohydrate Organisation (1987-2010)
- German Representative for International Carbohydrate Organisation (1990-2011)
- Dean Department of Chemistry (04/2002- 10/2005)
Editoral and Larger Projects
- Journal of Carbohydrate Chemistry - Regional Editor (1982-2012)
- Glycoscience - Chemistry and Chemical Biology - Coeditor (since 1998)
- Topics in Current Chemistry - Member of Editorial Board (since 1992).
- Speaker of the Collaborative Research Grant (SFB 470) "Glycostructures in Biosystems - Synthesis and Impact" (1997-2009)
- Part of the consortium of the collaborative EU project VIBRANT, funded by the European Union's Seventh Framework Programme (since 2009).
Research
Synthetic Carbohydrate Chemistry
In this area we are involved in a number of different projects. Some are concerned with methodology studies in glycosylation. In others we are elaborating applications for the synthesis of variously glycosylated natural products. Further studies deal with combinatorial glycosylations. Some recent work centered on the synthesis of fluorinated ketoheptoses as specific diagnostic agents (Fig. 1) [1,2].
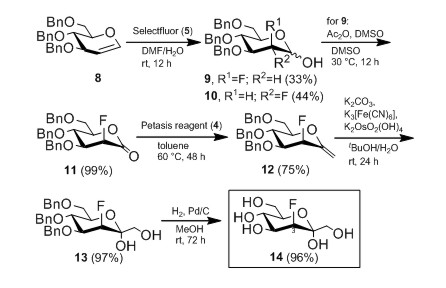
Figure.1: Synthesis of 3-deoxy-3-fluoro-D-manno-heptulose [1,2].
Selected Publications
[1] D. Waschke, Y. Leshch, J. Thimm, U. Himmelreich, J. Thiem Eur. J. Org. Chem. 2012, 948 – 959.
[2] D. Waschke, J. Thimm, J. Thiem Org. Lett. 2011, 13, 3628 – 3631.
[3] A. Schroven, S. Meinke, P. Ziegelmüller, J. Thiem Chem. Eur. J. 2007, 13, 9012-9021.
[4] S. Meinke, A. Schroven, J. Thiem Org. Biomol. Chem. 2011, 9, 4487 – 4497.
[5] M. Tober, T. Biemans, J. Thiem Farbe und Lack / Eur. Coatings J. 2012, 118, 16 – 19.
[6] M. Tober, J. Thiem Eur. J. Org. Chem. 2013, 566-577.
Chemoenzymatic Synthesis
Here we perform enzymatic synthesis employing efficiently available enzymes of the carbohydrate metabolism. In contrast to the analytical biochemical approach focus is rather on the preparative aspect. With much success hydrolases can be used for synthesis in the sense of transglycosylations. Also glycosyltransferases could be employed most efficiently giving rise to complex heterooligosaccharides . Recently some focus was on an investigation of the enzyme binding and inhibition of Trypanosoma cruzi trans-sialidase(TcTS). The novel phenyl-propyl a-C-glycoside of neuraminic acid proved to be the first carbohydrate-based modulator of this enzyme as shown in Fig.2 [3,4].
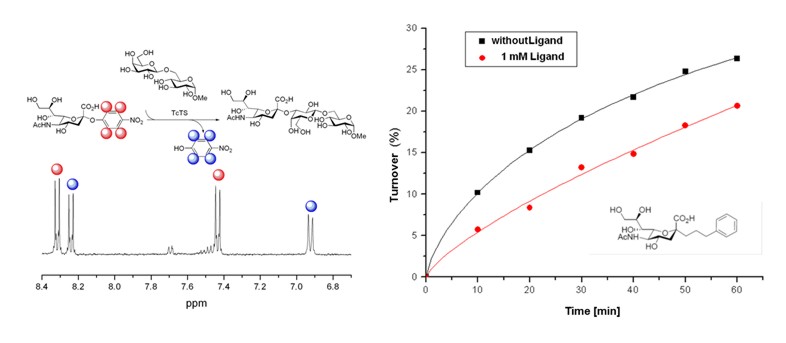
Figure 2: Transglycosylation progress monitored by NMR [4].
Selected Publications
[1] D. Waschke, Y. Leshch, J. Thimm, U. Himmelreich, J. Thiem Eur. J. Org. Chem. 2012, 948 – 959.
[2] D. Waschke, J. Thimm, J. Thiem Org. Lett. 2011, 13, 3628 – 3631.
[3] A. Schroven, S. Meinke, P. Ziegelmüller, J. Thiem Chem. Eur. J. 2007, 13, 9012-9021.
[4] S. Meinke, A. Schroven, J. Thiem Org. Biomol. Chem. 2011, 9, 4487 – 4497.
[5] M. Tober, T. Biemans, J. Thiem Farbe und Lack / Eur. Coatings J. 2012, 118, 16 – 19.
[6] M. Tober, J. Thiem Eur. J. Org. Chem. 2013, 566-577.
Carbohydrates as Chemical Feedstock
Formation of saccharide derived polymers requires avoiding the typical protecting group chemistry known for carbohydrate derivatives. We have realized the preparation of many polymers of interest such as polyesters, polyureas, polyurethane, polyamides and polyethers. Recently, carbohydrate-based oligohydroxy derivatives could be obtained which are useful as novel components for alkyd resin formation (Fig.3) [5,6].
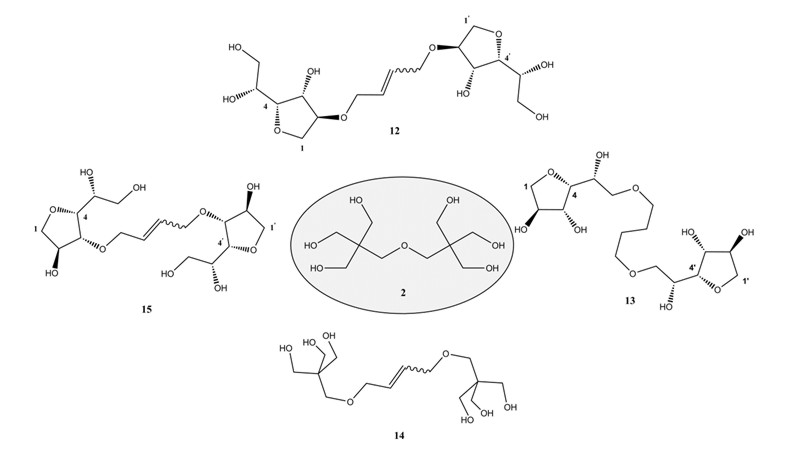
Figure 3: Dipentaerythritol and analogs obtained from regrowing ressourses [5].
Selected Publications
[1] D. Waschke, Y. Leshch, J. Thimm, U. Himmelreich, J. Thiem Eur. J. Org. Chem. 2012, 948 – 959.
[2] D. Waschke, J. Thimm, J. Thiem Org. Lett. 2011, 13, 3628 – 3631.
[3] A. Schroven, S. Meinke, P. Ziegelmüller, J. Thiem Chem. Eur. J. 2007, 13, 9012-9021.
[4] S. Meinke, A. Schroven, J. Thiem 2Org. Biomol. Chem. 2011, 9, 4487 – 4497.
[5] M. Tober, T. Biemans, J. Thiem Farbe und Lack / Eur. Coatings J. 2012, 118, 16 – 19.
[6] M. Tober, J. Thiem Eur. J. Org. Chem. 2013, 566-577.
