Main research areas
The research group Albert is researching the substitution of fossil-based chemicals with renewable raw materials. In collaboration with industrial and academic partners, it is working on alternative energy sources and basic chemicals in order to create a sustainable, bio-based foundation for future industrial development.
Catalytic valorisation of biomass - Teamleader Jan Krueger
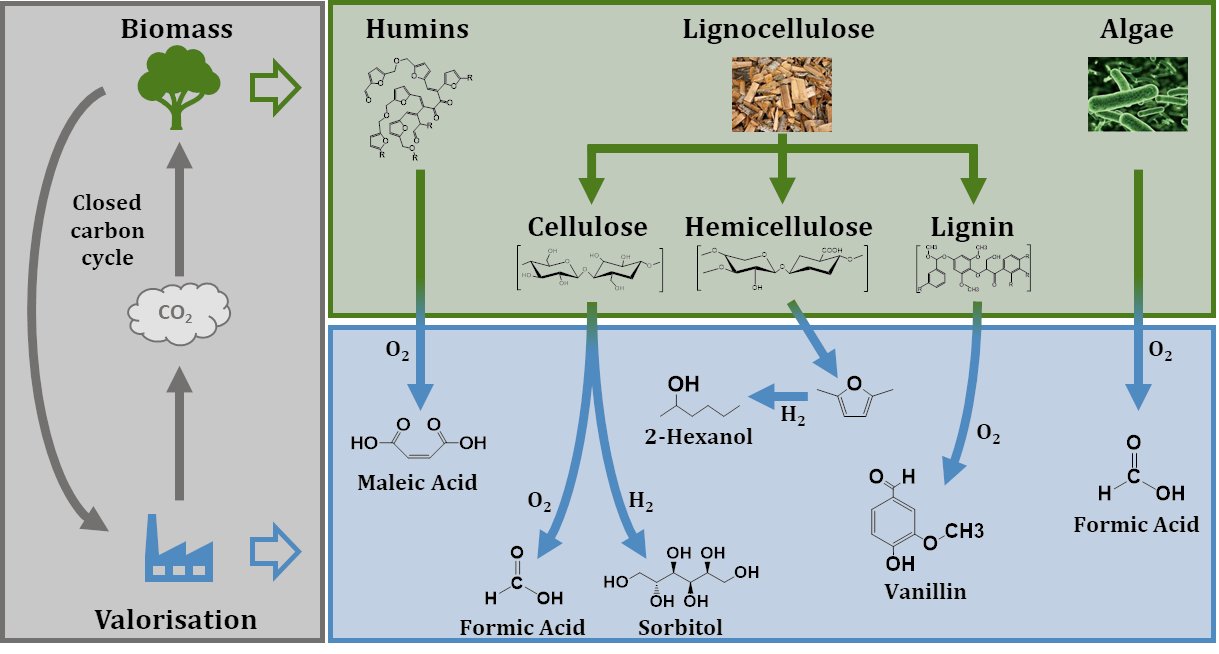
The chemical industry of the future will have to move from the use of fossil resources, such as coal, oil and gas, to more sustainable organic resources. Biomass offers great substitution potential among the renewable energy sources. Consequently, the interest in cost-effective biomass conversion processes for producing valuable platform chemicals is growing in recent years.
Lignocellulosic biomass as the most abundant class of biogenic materials typically contains more than 50 wt% sugars that can be upgraded to valuable platform chemicals. Lignocellulose consists of the three main components cellulose (ca. 50%), hemicellulose (25-30%) and lignin (15-25%). The aromatic lignin is the most under-utilised fraction. It has been traditionally used for heat and power purposes through combustion in the pulp and paper industry due to its high caloric value. However, it offers great potential for high-value chemicals like vanillin and its derivatives. Hemicellulose mainly contains C5 sugars like xylose or arabinose having important applications for biofuel production (e.g. bioethanol) and for the generation of valuable chemical intermediates (e.g. furfural). Cellulose is considered as one of the most abundant biopolymers on earth comprising linear β(1,4) glucose C6-chain links. Furthermore, the latter can be converted into valuable products such as biofuels and platform chemicals like formic acid, levulinic acid or lactic acid and derived products thereof, like several oxygenates.
Processing monosaccharides and their derivatives derived from cellulose, results in the co-production of dark-colored solids known as humins. These are insoluble in nearly all solvents but concentrated sodium hydroxide solution and cause several issues during biomass processing. It is reported that basic building blocks are most probably furan rings bridged by various aliphatic functional groups. According to literature, these furan rings can account for up to 60 % of the humin structure, while aliphatic linkers account for up to 20 %. Valorization of humins offers a great potential for cost-effective production of platform chemicals like maleic acid.
Algal biomass is an abundant renewable source of carbon (C) and nitrogen (N) combined within one species. Specifically, macroalgae represent a vast and underexplored resource for protein extraction, with benefits such as rapid growth rates and minimal environmental footprint. Recently, macroalgae proteins have gained significant scientific interest for application in non-food industries such as cosmetic and pharmaceutical sectors.
Contact: Jan Krueger, M.Sc. (jan-dominik.krueger"AT"uni-hamburg.de)
Chemical energy storage (Power-to-X) - Teamleader Nick Herrmann

Surplus electricity from renewable energies such as photovoltaics and wind power can be used to produce hydrogen sustainably by electrolysis. Due to the low volumetric energy density of hydrogen, technical storage by compression and liquefaction involves great effort, special materials and high costs. In particular, the long-term storage of large quantities of hydrogen is difficult and cost-intensive.
Alternatively, the regeneratively produced hydrogen can be fed into a further value creation process in a power-to-X application. Basic chemicals and fuels can be produced with CO2 from power plants, industrial waste gases and biogas plants. The production of e.g. methane, methanol, dimethyl ether or olefins under constant reaction conditions has been tested, but does not take into account fluctuating hydrogen supply from renewable energies.
Due to these dynamic conditions, novel, flexible and robust catalysts have to be developed to minimize undesired side reactions and catalyst deactivation. For process-technical evaluation, the catalysts will be investigated reaction-wise in a continuously controllable fixed-bed plant with fluctuating material flows and temperatures.
The choice of the optimal combination of active component and support material of the catalyst as well as its morphology is decisive for the catalytic performance in addition to the reaction conditions. For this purpose, promising power-to-X applications such as the (reverse) water-gas shift reaction, CO2 methanation, as well as methanol and dimethyl ether synthesis from CO2 are investigated under dynamic reaction conditions. These are coupled to each other via thermodynamic equilibria, so that by-products are inevitably formed. The goal of the research is to use a selective and robust catalyst to determine the optimal reaction parameters in each case to maximize the yield of the desired compound under fluctuating reaction conditions.
Contact: Nick Herrmann
E-Mail: nick.herrmann"AT"uni-hamburg.de
Nanomaterials for Catalysis - Teamleader Dr. Maximilian Poller
Catalysts are crucial in modern chemistry, as they lower the activation energy of reactions and enable more efficient, energy-saving processes. As society moves towards greater sustainability, their role in transforming the chemical industry has never been more important.
Our research supports the shift from fossil-based chemical processes to sustainable feedstocks such as green hydrogen, carbon dioxide (CO₂), and biomass. This transition marks a fundamental paradigm change—from assembling platform chemicals from simple hydrocarbons to managing the controlled breakdown of highly functionalized molecules found in biomass. Furthermore, while traditional processes are often oxidative, sustainable alternatives require more reductive strategies, especially when working with CO₂ and green hydrogen.
Developing new catalyst materials is essential for these next-generation chemical processes. In our team, we focus on the design, synthesis, and comprehensive characterization of innovative catalysts that are efficient, selective, and made from earth-abundant, non-toxic elements.
Through our work, we aim to enable greener chemical production and help drive the chemical industry’s transition to a more sustainable and environmentally friendly future.
If you are interested in learning more about our work or collaborating with us, please feel free to contact (maximilian.poller"AT"uni-hamburg.de)Dr. Maximilian J. Poller and the Catalyst Development team.
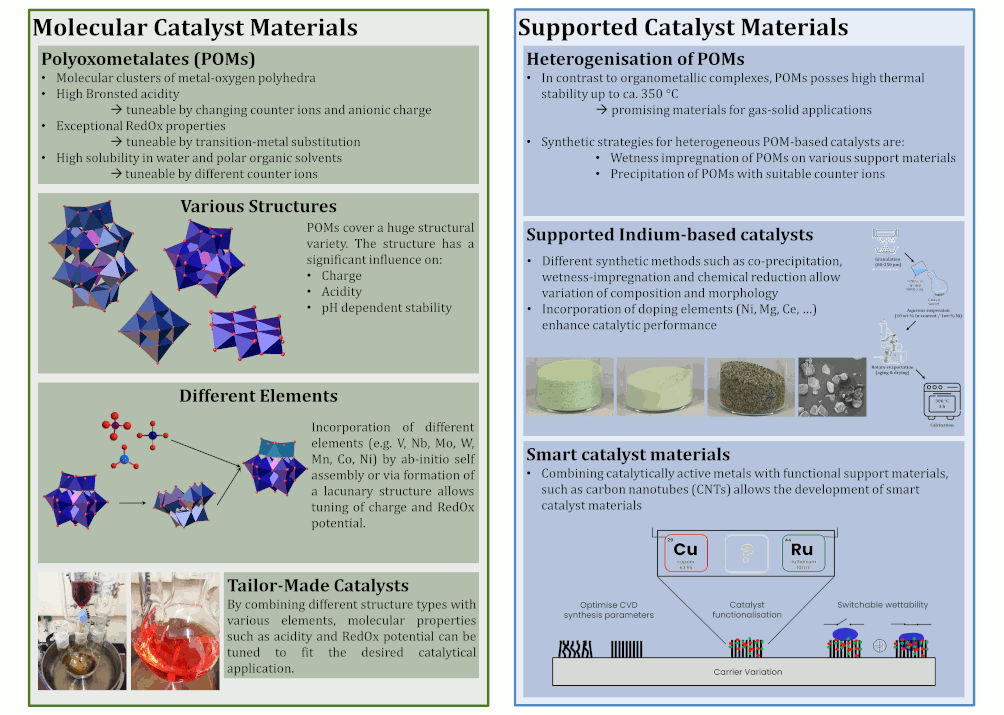
Scale-up and Miniplant technology - Teamleader Dr.-Ing. Dorothea Voß
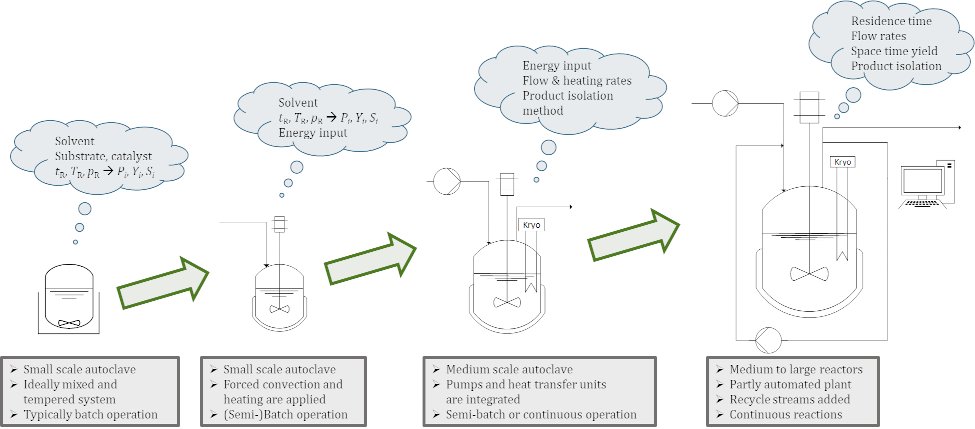
In the process of developing a chemical process to industrial stage, scale-up plays a crucial role. Here, technical chemistry forms the bridge between chemistry and process engineering. First, new synthesis strategies are developed in the laboratory (chemistry), followed by the safe transfer of the chemical conversion found in the laboratory to the technical scale (process engineering).
The miniplant technology forms the basis of scale-up. A miniplant is a small-scale laboratory or pilot plant with a throughput of 0.1-1 kg h-1. Building on discontinuous laboratory tests in which different influencing parameters such as pressure, temperature or catalyst systems are investigated, continuous investigations can be carried out in a miniplant for the first time. With the help of the miniplant, feasibility studies can be carried out and loops and recirculations can be tested. The interactions between the process units can also be investigated and the dynamic behavior as well as start-up and shut-down behavior can be studied. In addition, initial studies of long-term effects can be conducted. All in all, the miniplant represents an image of the later technical process. It is advantageous that standardized laboratory equipment (such as heat exchangers, pumps and reactors) can be used in miniplants. This makes them more space-saving, flexible and cost-effective than conventional pilot plants. In addition, equipment modifications can be carried out relatively quickly.
For scale-up, the design of the chemical reactors in terms of shape, size and mode of operation is just as important as the design of the separation concepts for the required production volume. The goal here is always to achieve the largest possible scale-up factor to save development time and costs. The process concept developed in the miniplant and the experimental results generated form the basis for a reliable scale-up to technical scale.

In our working group we investigate the scale-up of various processes. In the majority of our projects, after the optimization of the system, a scale-up is performed and an execution of the processes in a miniplant is aimed to be realized. In this context, we carry out all steps from the conceptual design, construction and implementation of the miniplants ourselves and also develop the necessary safety concepts for the safe operation of the miniplants.
Contact: Dr.-Ing. Dorothea Voß (dorothea.voss"AT"uni-hamburg.de)
