Completed projects
Here you will find an overview of all completed projects of the Albert working group.
Completed projects
Development of a concept for single-step dimethyl ether synthesis from CO2 and green hydrogen
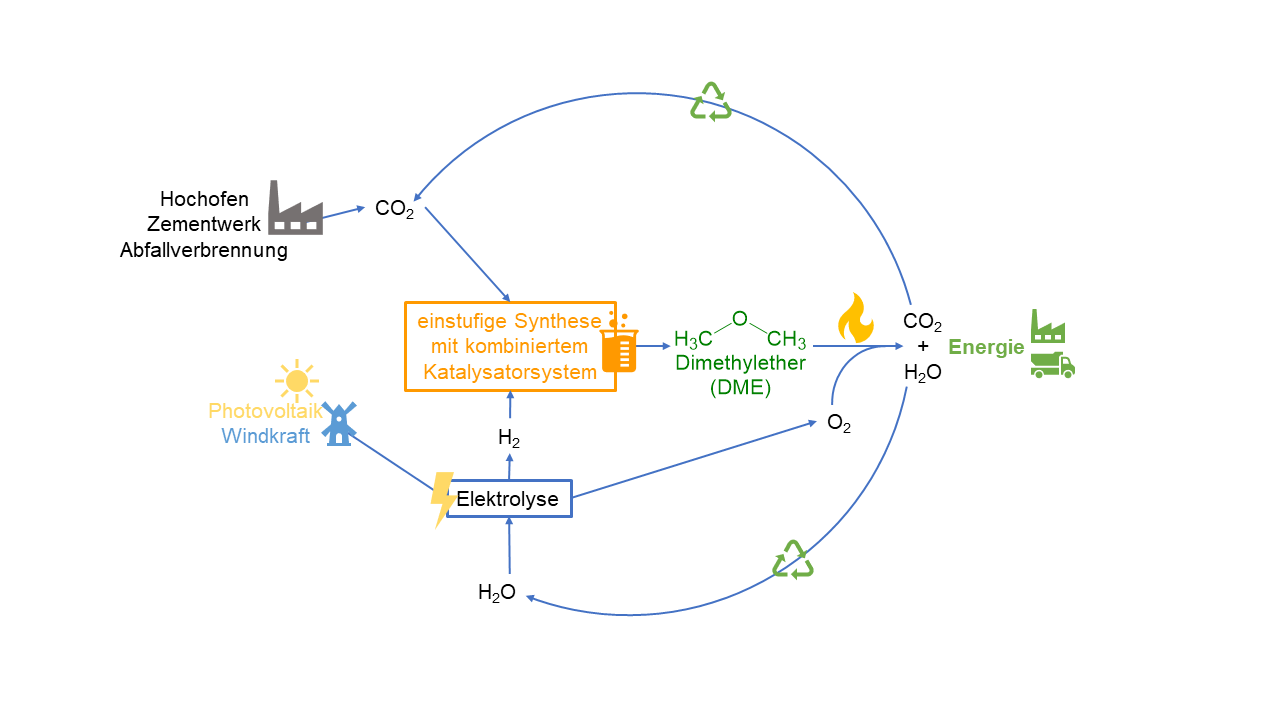
Both the struggle against advancing climate change and the current difficult geopolitical situation make it increasingly difficult to guarantee a reliable energy supply in Europe. In order to minimise our dependence on fossil fuels and the political powers that control them, our society is turning to renewable energy sources such as photovoltaics and wind power. However, the electrical energy generated in this way has the disadvantage that it is not always available and that it is difficult to store. This disadvantage can be compensated by using chemical energy storage concepts. For this purpose, the electrical energy, if it is available in surplus, is used to electrolyse water, producing hydrogen (H2). This "green hydrogen" can then be used to produce chemical energy carriers, such as dimethyl ether (DME).
DME has a boiling point of -24.8 °C, but can be liquefied at low pressure and can therefore be transported and stored in a similar way to conventional liquid gas (propane/butane). Its combustion properties make DME an promising alternative to diesel fuel. DME burns much cleaner than conventional diesel fuel, i.e. less particles, SOx, and NOx are emitted. This makes DME interesting as a sustainable fuel for construction machinery, for agricultural and forestry vehicles, or for disaster control/emergency services (vehicles, emergency power generators). Furthermore, DME can be used similarly to liquefied petroleum gas for heat generation, and its use in combustion power plants for electricity generation is also conceivable.
UHH/Albert
Currently, DME is produced from fossil raw materials in a multi-stage process. This process has many disadvantages, including its high energy demand. We want to solve these problems by applying a new catalyst concept and efficiently synthesising DME from CO2 and green hydrogen in a single step.
The use of CO2 to produce DME makes it possible to avoid the energy-intensive "steam reforming" of fossil raw materials. Upon combustion of the DME, only as much CO2 is released as was previously bound in it. This can then be recycled to avoid its release into the atmosphere. (Figure 1)
Within the framework of the project, we want to combine known methanol synthesis catalysts suitable for the reduction of CO2, with acid catalysts necessary for the formation of DME. The so-called heteropolyacids are particularly suitable for acid catalysis. These are molecular metal oxide compounds that exhibit a high Brönstedt acidity in their protonated form. In addition, they are characterised by a high thermal stability, which makes them suitable for use under process conditions up to 300 °C.
The aim of the project is to carry out the heterogeneously catalyzed methanol synthesis starting from CO2 and the subsequent methanol dehydratisation to DME in one process step. In this way, we want to develop a sustainable and environmentally friendly process for chemical energy storage.
This project is funded under the "Call4Transfer" funding programme by Hamburg Innovation GmbH in the period of April 2023 – March 2024.
Contact/Projekt leader: Dr. Maximilian J. Poller(maximilian.poller"AT"uni-hamburg.de)
Development of Indium based catalysts for Power-to-X Technologies
The development of new technologies for storing and transporting regeneratively generated energy and its surpluses is necessary to ensure a flexible energy supply.
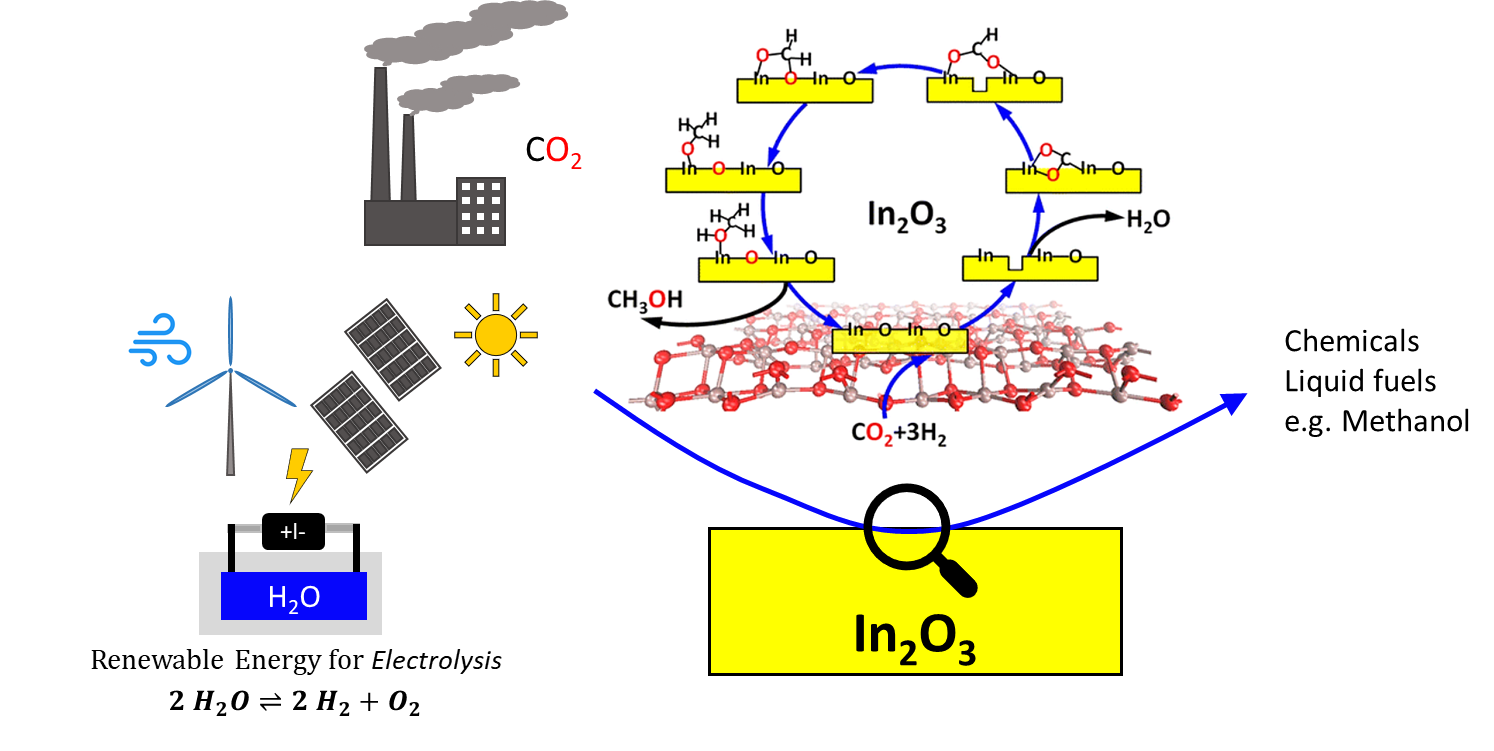
In this context, electrolytically produced hydrogen and its use in power-to-X processes is a potential solution. In this process, hydrogen is produced from water using renewable energies and then converted with CO2 to organic base chemicals or fuels (power-to-liquid). The required CO2 can be captured from the atmosphere or collected in industrial processes. Hydrogenation of CO2 to methanol shows promise for chemical energy storage applications:
CO2 + 3 H2 ⇋ CH3OH + H2O
Methanol is a major feedstock for the production of platform chemicals, such as formaldehyde, dimethyl ether, olefins, and acetic acid. Furthermore, methanol has great importance for the chemical and material processing industries.
A Cu/ZnO/Al2O3 system is currently used as a commercial catalyst for methanol synthesis from CO/CO2 mixtures. Under typical reaction conditions (T= 220-300°C; p< 100 bar), a conversion of XCO2 ≤ 30% is achieved, with selectivity towards methanol SMeOH varying from 30-70%. Indium-based catalysts represent a promising alternative. Under similar reaction conditions, they exhibit both improved CO2 adsorption capacity, higher methanol selectivity and increased long-term stability (Figure 1).
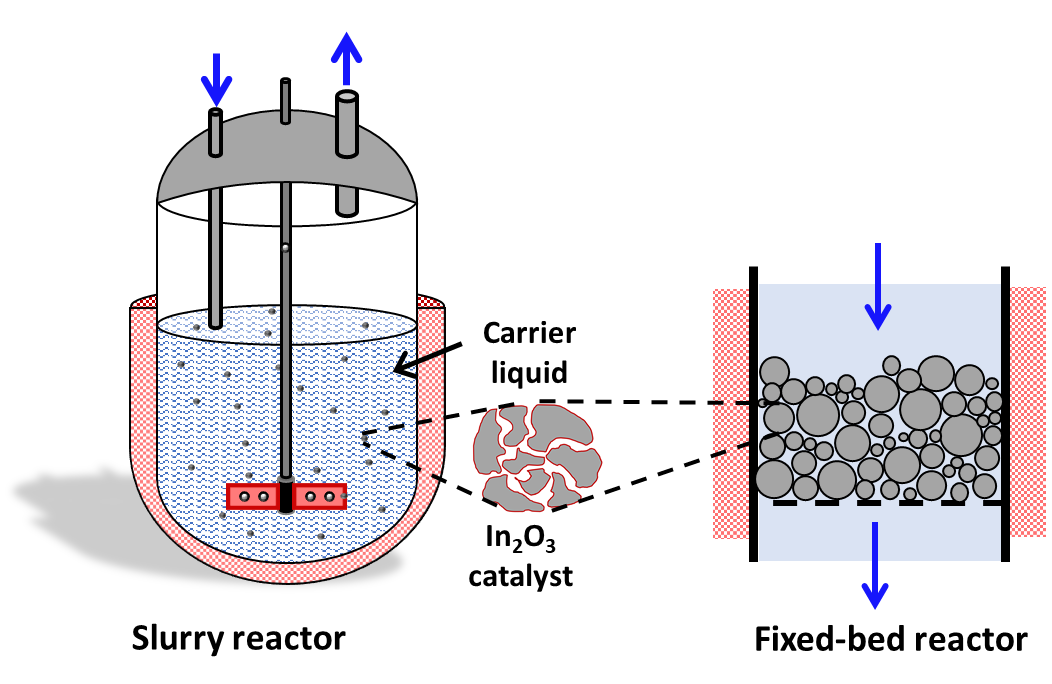
The aim of the project is to increase the performance and stability of indium-based heterogeneous catalysts by changing a wide range of parameters. By incorporating different dopants or applying In2O3 to different support materials, the possibility of synthesizing longer-chain hydrocarbons will be investigated. Testing will be carried out under near-industrial conditions in fixed-bed and suspension reactors (Figure 2).
In cooperation with the University of Duisburg-Essen, the promising synthesis method for heterogeneous catalysts is being further developed using pulsed laser ablation. Advantages such as a high purity of the synthesized materials and a cost-efficient scalability allow a targeted design of the supported In2O3 catalyst.
Contact: Anne Wesner, Philipp Kampe
Influence of N- and O-containing compounds on the continuous oxidative desulfurization of liquid fuels
Based on the current debate on pollutant emission, stricter environmental regulations for the combustion of liquid fuels are being adopted. Also for the nitrogen oxide and fine dust emissions of motor vehicles the environmental regulations became stricter. On the one hand, higher combustion temperatures have to be achieved in order to reduce particulate matter, but on the other hand, it produces more NOx. In particular, to observe the NO2 limit of 34 ppbw in the air, a nearly complete removal of nitrogen from liquid fuels is essential.
The currently used technology for nitrogen removal is based on the hydrogenation of nitrogen compounds using NiMo- or CoMo-fixed bed catalysts (Hydrodenitrification, HDN).
An alternative to the expensive HDN is the oxidative denitrification (ODN). The organic nitrogen compounds are oxidized by means of a suitable oxidizing agent and the corresponding catalyst to CO2 and N2. Catalyst systems suitable for this purpose are polyoxometalates. Typical reaction temperatures for ODN are 30 °C – 120 °C and thus significantly lower than for the HDN. Hydrogen peroxide is commonly used as an oxidant, but initial experiments with elemental oxygen show promising results, which provides a cheaper and greener alternative.
Our goal is to extend the already established system of Extractive Coupled Oxidative Desulfurization (ECODS) with the water-soluble polyoxometalate catalyst system HPA-5 (H8PV5Mo7O40) to the oxidative removal of other heteroatoms such as nitrogen and oxygen. For this purpose, indoles and furans, as well as their derivatives are used as model compounds. In addition to the characterization of the reaction products, influences such as matrix effects or fuel mixtures should also be investigated. In addition, a simplification of the catalyst system consisting of HPA-1/VOSO4 is to be investigated.
This project is being worked on together with the group of Prof. Jess at the Chair of Chemical Engineering at the University of Bayreuth. Furthermore, we cooperate closely with the working group of Dr. Skiborowski of TU Dortmund in the field of product purification. This project is generously funded by the Deutsche Forschungsgemeinschaft (DFG) under funding code AOBJ: 655412 over a period of three years.
contact person: Michael Huber
Molecular Nanomaterials (Polyoxometalates)
Polyoxometalates (POMs) constitute a versatile class of molecules with a wide range of applications. In our research, we focus on harnessing their unique properties for catalytic processes, particularly for the valorisation of biomass and the development of sustainable chemical transformations.
POMs are anionic molecular clusters composed of transition metal ions (such as Mo+VI or W+VI) bridged by oxo ligands (O2–). They exhibit a variety of structure types, broadly categorized into isopolyanions (containing only the metal and oxo ligands) and heteropolyanions (which additionally incorporate a heteroatom such as silicon or phosphorus). Both structure types can be further modified by partially substituting the framework metals with other transition metal ions (e.g., V+V, Nb+V, Co+II, Ru+III), allowing us to fine-tune their catalytic properties. Beyond structural variation and elemental substitution, the properties of POMs—particularly their solubility and reactivity—can also be controlled by carefully selecting their counterions.
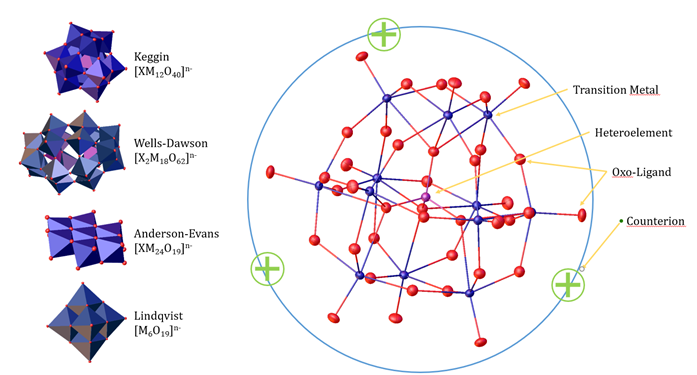
Our team develops synthesis methods enabling us to specifically tailor POM characteristics—including structure type, composition, and counterion selection—to achieve desired molecular properties such as solubility, Brønsted versus Lewis acidity, and redox activity. Using these approaches, we create bespoke POM-based catalysts whose catalytic activity is systematically studied in diverse projects geared towards sustainable chemical processes.
Depending on the application, we use POMs either as homogeneous catalysts in solution or immobilized on support materials as heterogeneous catalysts. Through this research, we aim to exploit the full potential of POMs for innovative and sustainable chemical transformations.
Contact: Pegah Saedi (pegah.saedi"AT"uni-hamburg.de)
Biogenic formic acid as sustainable hydrogen carrier
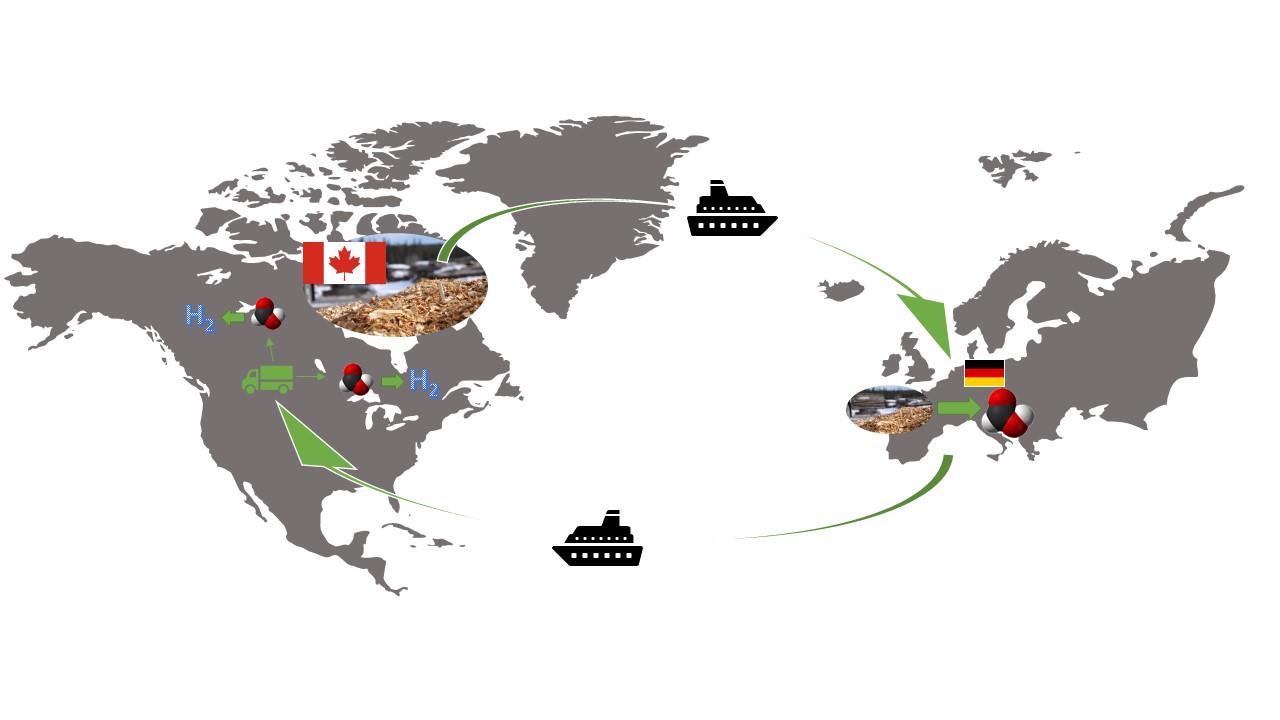
In the context of the German national hydrogen strategy as well as a Call of Action of the Canadian government, similar goals for the development of a hydrogen economy were set. In cooperation with McMaster University in Hamilton, this project will evaluate the usability of biogenic formic acid as a hydrogen storage molecule. Hydrogen stored in the gaseous state means compression to 700 bar with an energy density of 5.0 MJ/L (comparatively gasoline 32 MJ/L). This approach is expensive, energy-intensive and involves a safety risk, since hydrogen forms an explosive gas with oxygen. Therefore, research is being conducted on alternative safe storage options for hydrogen. Formic acid is chemically composed of two hydrogen atoms together with one carbon atom and two oxygen atoms (HCOOH). It can be converted to hydrogen and CO2 as well as CO and water, therefore formic acid can be used as a hydrogen storage as well as a syngas equivalent (H2/CO). It is possible to produce formic acid sustainably by converting renewable raw materials (e.g. wood) using polyoxometalates (OxFA process). Formic acid is liquid at room temperature and atmospheric pressure, non-toxic and accordingly easy to handle. Formic acid has a 20% higher energy density than hydrogen (6.4 MJ/L). Due to the sustainable production of formic acid, the entire process can be considered CO2 neutral.
Through close collaboration between our research group and the research group of Prof. Adams II at McMaster University Hamilton, Canada, an ecological, sustainable production route of formic acid as a green hydrogen carrier from Canadian wood chips will be investigated. This will involve investigating three steps: production of formic acid from renewable resources, storage and transport of formic acid, and recovery of hydrogen from formic acid. Our research group will be involved in the laboratory experiments and the practical implementation of the different steps. The research group of Prof. Adams II will deal with the theoretical simulation of scale-up concepts and the evaluation of the process using a Life Cycle Assessment (LCA).
The project "Biogenic Formic Acid as a Sustainable Hydrogen Carrier (BioFA)" is funded by the German Federal Ministry of Education and Research (BMBF) as part of a German-Canadian cooperation in the funding area "Mobility with Canada 2021" starting 01.10.2021.
Contact: Stefanie Wesinger
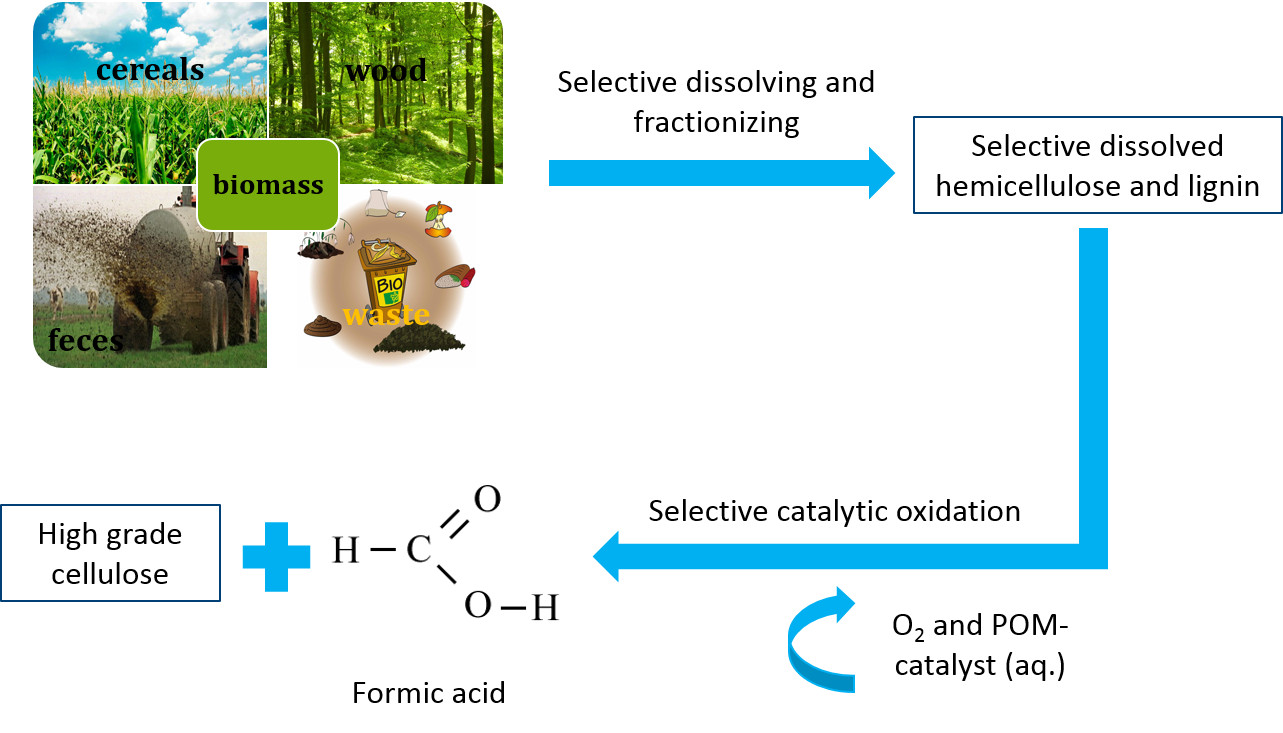
Fractionation and selective oxidation of lignocellulosic biomass
Lignocellulosic biomass is one of the most important renewable resources for the sustainable production of biofuels, bio based materials and platform chemicals. Lignocellulosic biomass is generated by atmospheric CO2, water and sunlight via photosynthesis and can therefore be regarded as a promising alternative to fossil resources with zero netto CO2 emissions that can be provided sustainably in large quantities. However, gaining value from lignocellulose is more challenging due to the higher complexity of the raw material and its higher recalcitrance towards selective processing. It typically consists of hemicellulose (25 %), lignin (25 %), cellulose (40 %) and ca. 10 % other, minor components.
Cellulose is an attractive product for material applications like paper, while hemicellulose and lignin can be used for energy generation or the production of bulk chemicals. Due to the higher value of cellulose compared to hemicellulose and lignin, there are several approaches for fractionation of lignocellulosic biomass into its main components. This fractionation facilitates the selective use of cellulose for the paper industry and the further processing of lignin and hemicellulose for the production of bulk chemicals.
Formic acid (FA) is an important bulk chemical that is widely used in chemical, leather, pharmaceutical, rubber and other industries. Furthermore, FA can be easily and selectively decomposed to hydrogen and CO2 under mild reaction conditions. Hence, FA can be regarded as an attractive hydrogen storage material.
In this context, our working group investigates the fractionation of lignocellulosic biomass and selective in-situ conversion of hemicellulose and lignin to formic acid while cellulose remains untapped for further processing. For these approaches, several tailored polyoxometalate-catalysts as well as liquid reaction matrixes are used. Hereby, we collaborate with several partners from academia (Imperial College London) as well as industry (Chrysalix, UK and OxFA GmbH, DE).
contact person: Anna Bukowski
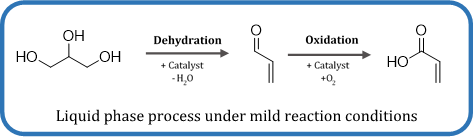
Process development for the selective production of acrylic acid from glycerol
The sustainable production of platform chemicals is one of the most present challenges of the chemical industry nowadays. One of those relevant platform chemicals is acrylic acid (AA). Due to the presence of two functional groups within this molecule acrylic acid shows a high reactivity and therefore a wide application potential. AA can be converted to several acrylic esters, acrylic chlorides and can be easily polymerized to form e.g. polyacrylic acid (PAA). Those acrylates and PAA can be e.g. used as plastics, synthetic rubbers, surface coatings and in the cosmetic industry. Hereby, especially the moisture absorbing properties of PAA are of high interest. Therefore, PAA as “superabsorbent polymers” are mainly used as moisture absorbing material in disposal diapers.
Nowadays, acrylic acid is mainly produced via oxidation of propene. Disadvantages of this fossil based process are e.g. the dependence on fossil resources, the high temperatures needed, a complex downstream processing and the need for storage of hazardous intermediates.
In cooperation with our partner Nitto Denko, we are developing an alternative liquid phase process for the production of acrylic acid with glycerol as a substrate. Glycerol is produced as a byproduct (3.5 MioT/a) at the biodiesel production. Therefore, glycerol is easily available and produced based on renewable resources.
Within our project we are developing an innovative system for the sustainable production of acrylic acid under mild reaction conditions.
Catalyst development using reaction medium-optimized polyoxometalate catalysts for the sustainable production of fuels
Formic acid and its esters (formates) are important platform chemicals used in various industries. In the Erlangen OxFA process, formic acid is produced from different biomasses in water under very mild conditions with high product selectivity using polyoxometalates. However, if this process is carried out in methanolic solutions, the combined formic acid yield can be significantly increased. The main product methylformate (methyl ester of formic acid) can easily be separated by distillation. In the main project in cooperation with the Helmholtz Institute Erlangen-Nuremberg, short-chain polyoxymethylene dimethyl ethers (OME3-5) are to be produced sustainably from biogenic formic acid or biogenic methyl formate. These OMEs can be used as an emission-reducing fuel additive and cause a significant reduction in NOx and particle emissions.
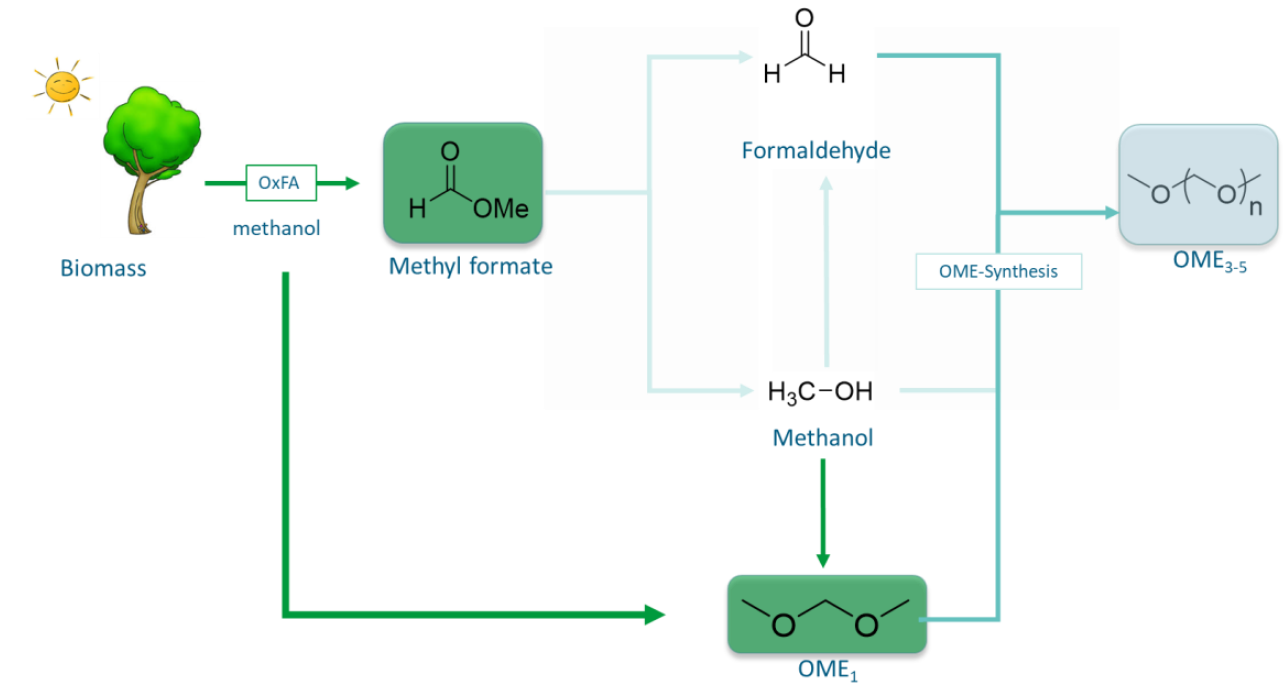
A catalyst optimization has not been carried out for the alcoholic process yet. For this purpose, the effect of the catalyst in interaction with the alcoholic reaction medium used in the reaction will be examined. As a result, a structural and chemical adjustment of the catalyst should take place, to increase efficiency and selectivity. This optimization promises a sudden increase in performance of the modified process, especially with regard to the reaction rates at reduced oxygen partial pressures. In cooperation with the Helmholtz Institute Erlangen-Nuremberg, the optimized process conditions and catalysts will be transferred to a continuous process with the help of micro reaction technology.
Contact person: Jan Krueger
E-Mail: jan-dominik.krueger"AT"chemie.uni-hamburg.de
Lignoanalytics
Analysis and Consulting on Technical Lignin for Biorefinery
In order to make our chemical industry more sustainable, it is necessary to move away from fossil raw materials and to utilize renewable raw materials instead. A very promising raw material is lignocellulosic biomass. However, while the cellulose content is already widely utilized in the form of paper and related materials, the lignin content is so far treated as waste. For a more sustainable usage of the raw material it would be greatly beneficial to utilize these lignin waste streams from the pulp and paper industry. This is of particular interest because lignin is a potential sustainable source for aromatic compounds, which are so far exclusively sourced from fossil resources. To this end, we are exploring the chemical composition of various lignin samples from different technical processes with the goal of helping our partners from the H&R group to evaluate their suitability for use in biorefineries.
LigPOMMem - Complete valorization of lignin to C1-C6 chemicals in a catalytic membrane reactor
The research project aims to convert technical lignin streams into C1-C6 chemicals in a catalytic membrane reactor as C-efficiently as possible.
The production of platform chemicals from biomass is a central concern of the chemical industry and ultimately of society. Existing processes are to be converted from fossil educts to renewable raw materials. Based on preliminary work, an integrated reaction concept for the depolymerization of lignin into C1-C6 chemicals will be developed.
The focus here is on the following product fractions in particular:
- C1-, C2- fraction: formic and acetic acid
- C3-, C4- (di)-carboxylic acids
- C1 – C6 aldehydes, acids (+ esters), ketones
These are to be produced oxidatively from lignin in a catalytic membrane reactor and separated in-situ from the reaction solution using nanofiltration membranes.
This project is funded by LigPOMMem GmbH and focuses on the selection and fluid mechanical and process engineering characterization of suitable membranes. On the basis of the knowledge gained, a catalytic membrane reactor will then be designed, constructed and commissioned. Catalytic studies on the oxidative depolymerization of lignin in the catalytic membrane reactor will then show the potential of the new concept in comparison to established lignin value chains.
Contact: Dr.-Ing. Dorothea Voß(dorothea.voss"AT"uni-hamburg.de)
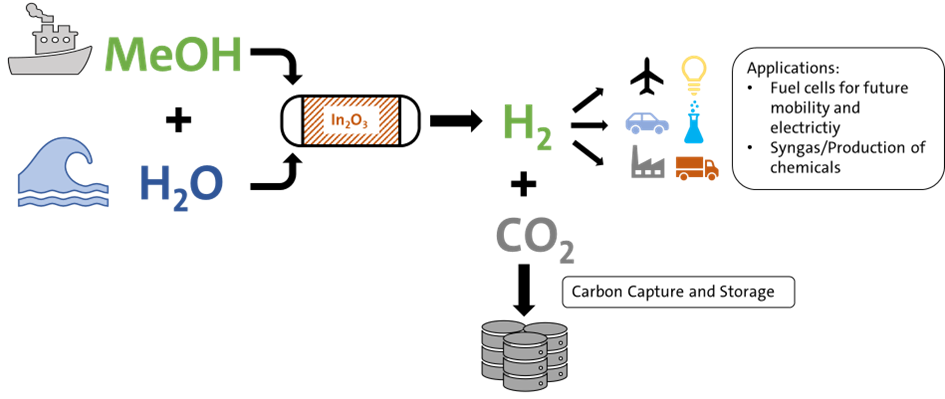
MSR - Methanol Steam Reforming using Indium-based catalysts
Methanol Steam Reforming using Indium-based catalysts
UHH/Herrmann
Methanol is an excellent hydrogen carrier liquid, is easy to store and to transport without loss over long distances, and has a higher volumetric energy density than pure hydrogen. It has a high hydrogen to carbon ratio, lacks carbon-carbon bonds and can release hydrogen at mild conditions. In addition, methanol can already be transported using our current petroleum infrastructure. This allows energy to be transferred from areas with a lot of renewable energy to areas where a lot of hydrogen is needed and cannot be produced locally. This includes heavy industry and the energy needs of entire countries, being readily connected with ports and pipelines. Without, a lot of fossil fuels will still be needed to cover the energy demand of the latter.
The Methanol Steam Reforming (MSR) reaction is an important link to provide large amounts of hydrogen for energy and industrial processes. With adding steam to the reforming, the hydrogen bound in water can be released as well, increasing the overall hydrogen yield. In previous research, indium-based catalysts supported on zirconia have shown good results in the synthesis of green methanol using hydrogen and CO2 and may be suitable for the MSR reaction as well.
Different indium-based catalysst will be tested for their activity towards MSR in a dynamically operating fixed bed reactor. Subsequently, the catalyst will be tuned and the reaction conditions optimized, such as temperature, pressure, Steam/Methanol and more.
Contact: Nick Herrmann
Email: nick.herrmann"AT"uni-hamburg.de
POM for MELiSSA (in Cooperation with ESA)
In the context of long-term manned exploration missions, regular resupply of the crew with the necessary metabolic consumables becomes technically and economically unfeasible. Therefore, the capability of Life Support Systems (LSS) will have to increase. In addition to air revitalisation and water recovery functions, future LSS will also include waste recycling and food production functions. Such a LSS is known as a closed loop LSS, which MELiSSA is an example of. MELiSSA aims at the recovery of oxygen, water and food through transformation of organic wastes generated during a manned space mission. The waste stream considered by the MELiSSA loop is mostly composed of ligno-cellulosic materials originating from the production of higher plants and solid metabolic wastes produced by the crew.
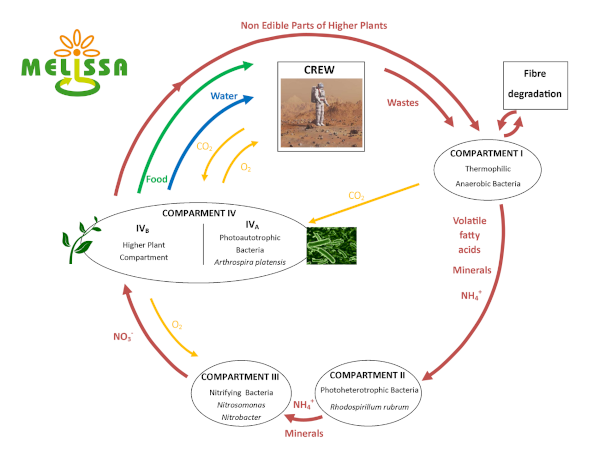
Complete transformation of ligno-cellulosic materials into formic acid and CO2 (OxFA process) under mild temperatures and oxidative atmosphere have been demonstrated using polyoxometalates (POMs) as a catalyst. It has also been demonstrated that POMs can be used for multiple oxidation cycles with little impact the oxidation efficiency. The use of less selective POMs (e.g. Keggin-type POMs) have the potential to favour the production of CO2 instead of formic acid from lingo-cellulosic material. This possibility needs to be explored.
In the context of this project the feasibility of achieving full oxidation of carbon contained in the ligno-cellulosic part of the MELiSSA waste stream will be assessed as well as the feasibility of achieving full oxidation of the volatile fatty acids produced by the MELiSSA waste compartment. The recovery of minerals (e.g. nitrate, sulphate and phosphate) from the reaction broth is also being investigated.
contact person: Stefanie Wesinger
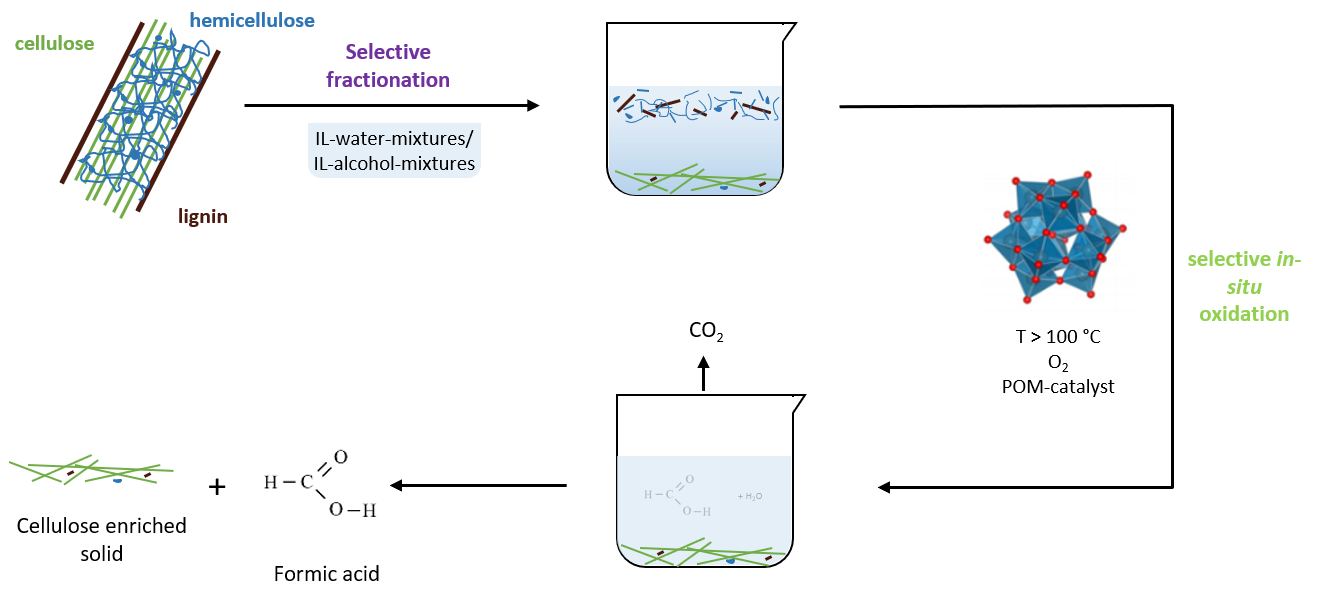
POM-Ionosolv-Concept
Lignocellulosic biomass is one of the most important renewable resources for the sustainable production of biofuels, bio based materials and platform chemicals. Lignocellulosic biomass is generated by atmospheric CO2, water and sunlight via photosynthesis and can therefore be regarded as a promising alternative to fossil resources with zero CO2 emissions that can be provided sustainably in large quantities. However, gaining value from lignocellulose is more challenging due to the higher complexity of the raw material and its higher recalcitrance towards selective processing. It typically consists of hemicellulose (25 %), lignin (25 %), cellulose (40 %) and ca. 10 % other, minor components.
Cellulose is an attractive product for material applications like paper, while hemicellulose and lignin can be used for energy generation or the production of bulk chemicals. Due to the higher value of cellulose compared to hemicellulose and lignin, there are several approaches for fractionation of lignocellulosic biomass into its main components. This fractionation facilitates the selective use of cellulose for the paper industry and the further processing of lignin and hemicellulose for the production of bulk chemicals.
Formic acid (FA) is an important bulk chemical that is widely used in chemical, leather, pharmaceutical, rubber and other industries. Furthermore, FA can be easily and selectively decomposed to hydrogen and CO2 under mild reaction conditions. Hence, FA can be regarded as an attractive hydrogen storage material.
In this context, our working group investigates the fractionation of lignocellulosic biomass and selective in-situ conversion of hemicellulose and lignin to formic acid while cellulose remains untapped for further processing. For these approaches, several tailored polyoxometalate-catalysts as well as liquid reaction matrixes are used. Hereby, we collaborate with our academic partners from Imperial College London.
Contact:
- Stefanie Wesinger
E-Mail: stefanie.wesinger"AT"chemie.uni-hamburg.de
Tel.: +49 40 42838 – 6047
Selective hydrogenation of biomass derived compounds to biofuels using polyoxometalate catalysts
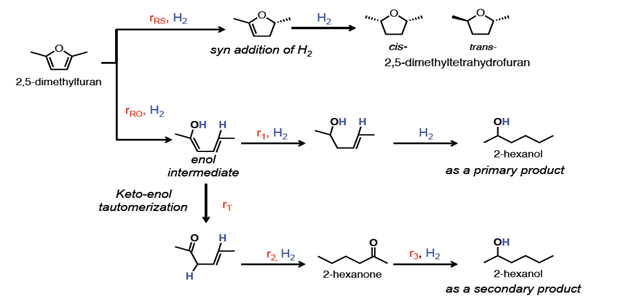
In order to increase the material value added from biomass, which is the only renewable carbon source available, secondary energy sources derived from biomass are converted into higher-quality platform chemicals by hydrogenation in the proposed research project. For this purpose, the bio-derived compounds dimethylfuran, methylfuran, and furan in combination with renewable hydrogen from i.e. electrolysis will be hydrogenated to bio-hexanol, bio-pentanol and bio-butanol, respectively. Those alcohols have a great importance in many industrial branches.
In this project, a benchmark using commercial catalysts will be tested for the hydrogenation reaction of those biomass-derived compounds at first, then various polyoxometalate structures should be synthesized and used as selective hydrogenation catalysts for the production of the above mentioned bio-alcohols. These bio-alcohols are used as solvents for the extraction of essential oils, natural resins, dyes and antibiotics, as well as a component of hydraulic and brake fluids. In addition, they are considered as potential biofuels that can replace diesel and gasoline fuels.
This project is founded by DAAD since April 2019 in the form of a German Egyptian Research Long-term Scholarship (GERLS).
contact person: Magdy Sherbi
Sensor for e-fuels
In order to achieve the climate target, CO2-neutral drive concepts in the transport sector, among other things, are appropriate. In addition to electric vehicles, biomass-based fuels and e-fuels offer high potential for CO2 reduction. The use of renewable fuels in gasoline, diesel or hybrid vehicles can create a closed carbon cycle, as the CO2 released during combustion is removed from the atmosphere again to produce the fuels.
Longer transport distances for renewable fuels, such as various alcohols, can lead to fuel aging, and undesired by-products can be created.
One aim of the project, which is being carried out in cooperation with Coburg University of Applied Sciences, is the mass balancing of aged fuels. For this purpose, various fuels are thermally oxidized and analyzed after aging. In this way, the various reaction mechanisms of the aging processes of several fuels are to be developed and the aging products quantified in the liquid phase and in the gas phase.
Furthermore, a sensor concept is being developed to determine the degree of aging of various regenerative fuels, as well as the fuel parameters required for their use. This is to ensure that the physical properties do not change too much during aging due to the formation of further aging products.
The project is funded by the Forschungsvereinigung Verbrennungskraftmaschinen e.V. (FVV) under the funding code 6013424.
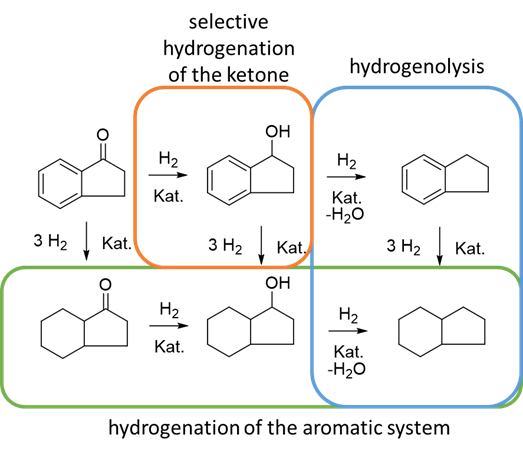
Selective hydrogenation of 1-indanone over Pt/SBA-15 catalyst in a slurry reactor
Selective hydrogenation of 1-indanone over Pt/SBA-15 catalyst in a slurry reactor
The selective reduction of unsaturated aromatic carbonyl compounds into the corresponding alcohols is of great importance for the development of fine chemicals. For the reduction of carbonyls, strong inorganic reducing agents such as LiAlH4 or NaBH4 are deployed. These show (depending on the reaction conditions) a high selectivity and are mainly used in the syn-thesis of organic fine chemicals. Due to the poor atomic economy and the difficulty of han-dling, their use on a larger scale is limited.
The reactions are to be carried out in a slurry reactor with high-pressure hydrogen in a heter-ogeneous three-phase reaction. In cooperation with the Fraunhofer-Institute, new catalysts and materials are available to the working group. Those are to be investigated using the model reaction in the course of the master's thesis. The objective is to investigate the influence of different catalysts on the selectivity of the hydrogenation using the model substance 1-indanone.
UHH/Herrmann
Different methods for the product analysis such as GC-MS, HPLC and NMR are available. For a closer analysis of a promising catalyst ICP-OES, XRD, TEM as well as H2-TPR can be used.
Contact: Nick Herrmann
Email: nick.herrmann"AT"uni-hamburg.de
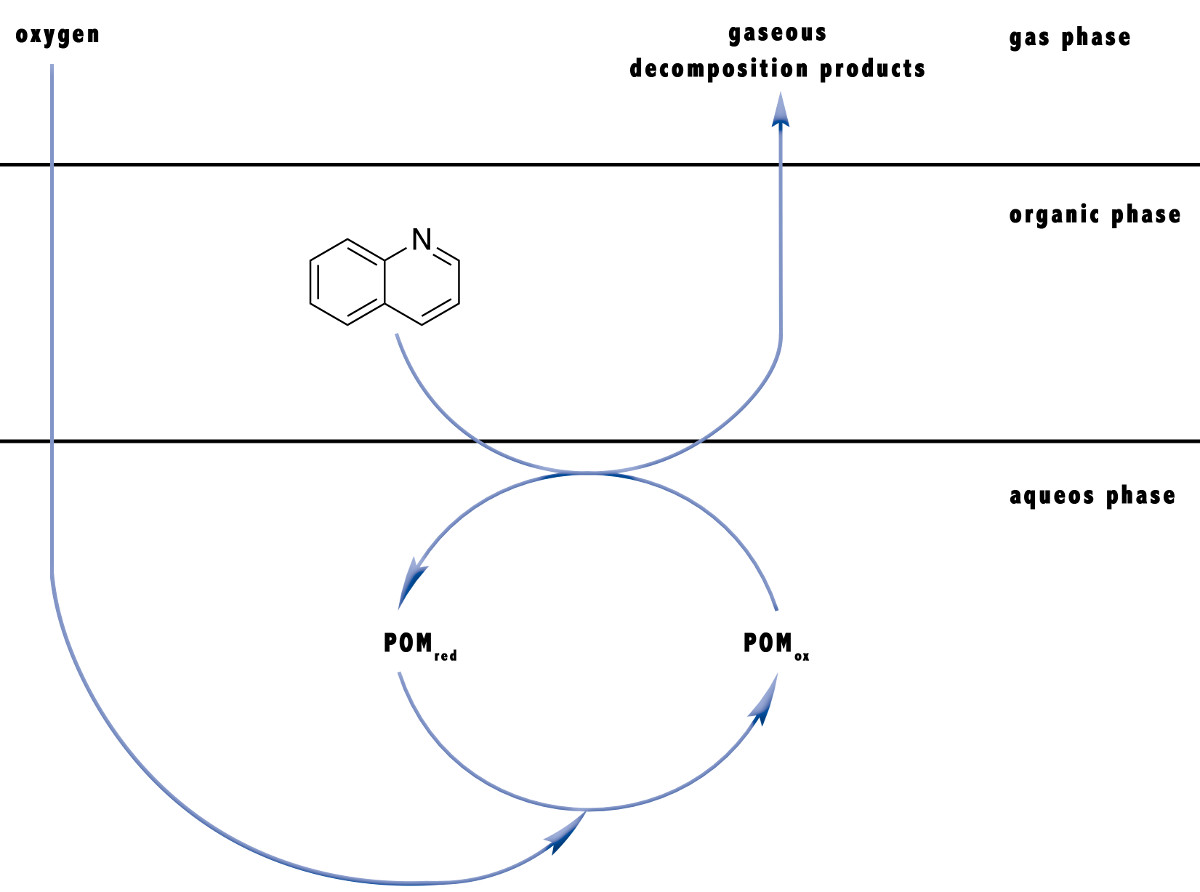
Oxidative catalytic valorisation of biomass using a jet loop reactor
The production of platform chemicals from biomass is a central concern of the chemical industry and ultimately of society. The aim is to convert existing processes from fossil reactants to renewable raw materials. An exemplary process for this is the OxFA process, in which biomass is oxidatively converted into formic acid and CO2 with the aid of a catalyst in water. The catalyst must be continuously oxidized in the course of the reaction, as it is only active in its oxidized form. This reoxidation takes place in the aqueous phase with molecular oxygen, which must be introduced into the reaction medium.
This multiphase reaction is mass transfer limited, since the mass transfer of the oxygen into the aqueous phase is slower than the oxidation of the substrate used. This limitation must be overcome in order for the reaction to proceed faster. A promising reactor for this task is a jet loop reactor (Figure 1).

UHH/Albert
In this reactor, the liquid in the reactor is sucked out of the bottom of the reactor by a pump and returned through a nozzle at the top of the reactor. The required oxygen is fed through the nozzle by a capillary and exits at the nozzle outlet. The efficient circulation of the reaction medium ensures an extremely homogeneous concentration and temperature distribution in the reactor.
Contact: Daniel Niehaus
E-mail: daniel.niehaus"AT"uni-hamburg.de
SMART catalysts
Supported Ru-X on carbon nanotubes as switchable catalyst for glycerol hydrogenolysis
The aim of CRC 1615: SMART-reactors is to build a reactor that converts renewable resources into different products (multipurpose) and that operates autonomously (self-adaptive). This will lead to more resilient processes that are better transferable between scales and locations. Designing tailor-made bi- or tri-metallic catalysts for the selective hydrogenolysis of glycerol, a major byproduct of biodiesel production, into 1,2-PD under mild reaction conditions is a key challenge for the project. Given that the catalyst's wettability should be switchable to make it more resilient against catalyst poisons, carbon nanotubes seem to be a promising support. To achieve this, we aim to develop a highly active and selective metallic catalyst on a tailor-made CNT-forest, whose wettability can be tailored by applying voltage.
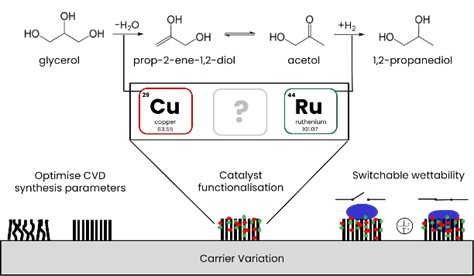
Preliminary tests performed by our group showed that the combination of transition and noble metals is a promising approach. As ruthenium seems to be the best choice for the noble metal, finding the right transitional metal to complement the bi- or tri-metallic catalyst is a key step in our research. It is also important to find a suitable CNT support that further enhances catalytic activity. For that reason, we use different commercially available CNTs for the development of a powder catalyst. We want to find out if the properties crucial for catalysis in the tested powder CNTs are transferable to tailor-made CNT-forests from our project partners. It is also important to find the optimal metal ratio, loading, precursors, impregnation method, and conditions to develop the best possible catalyst. We test our catalysts in a multi-fold hydrogenation plant, which allows us to test our catalysts under different reaction conditions. This project is part of CRC 1615 SMART-reactors and is funded by the German Research Foundation (DFG) under the funding number 503850735. We work in close cooperation with our project partners from the working group of Prof. Dr.-Ing. Bodo Fiedler at the Institute of Polymers and Composites at TU Hamburg.
Contact: samrin.shaikh@uni-hamburg.de
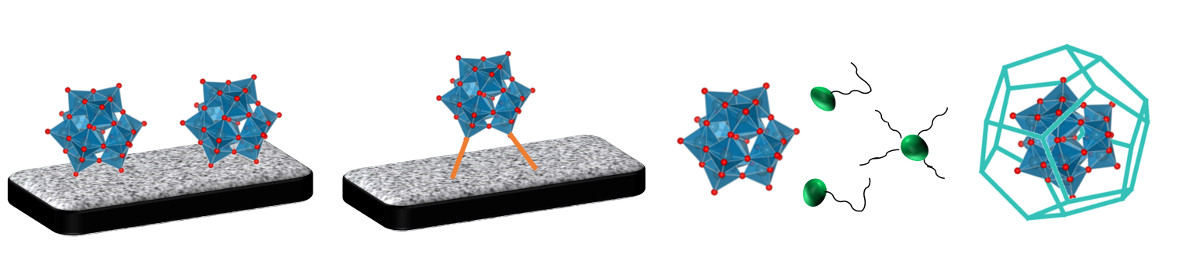
Development and application of heterogeneous POM-based catalysts
Polyoxometalates (POMs) are well-defined metal-oxyanions linked with oxygen bridges of early transition metals of the main groups 5 or 6 at their highest oxidation state. They can also contain a multitude of hetero anions like phosphate or silicate to improve their chemical and thermal stability. POMs have attracted considerable attention due to their fascinating architectures and attractive physico-chemical properties including strong Brønsted acidity, high proton mobility, fast multi-electron transfer, high solubility in polar solvents and resistance to hydrolytic and oxidative degradations. Many properties of POM materials can be tailored by changing the constituents and counter cations. Especially the enormous multifunctionality of POMs made them in particular attractive for various homogeneous catalysed applications.
However, homogeneous catalysis leads to several drawbacks like difficulties in catalyst separation and recycling as well as purification of the products. Furthermore, the low specific surface area of bulk POM-catalysts limits the field of application. Therefore, the development of heterogeneous POM-based catalysts with higher specific surface area has attracted much attention. Beside a better catalyst separation and catalyst recycling, especially extending the field of applications of POM-based materials to liquid-phase reactions in non-polar solvents and gas-phase reactions is one of the main goals.
There are several possibilities for heterogenisation of polyoxometalates:
- Impregnation on a porous support (via physical adsorption)
- Chemical immobilization via grafting on a porous support (covalent bondings using linker molecules)
- Complexation with organic or inorganic cations
- Encapsulation in highly porous frameworks
In our research group we are developing tailored heterogeneous POM-based materials using those heterogenisation methods for technical liquid phase and gas-phase applications.
We are working on this project together with our academic partners at Lyngby Technical University (DTU).
contact person: Maximilian Poller
SNISMs
SNISMs- Combined influence of pH, catalyst and strongly non-ideal solvent mixtures towards boosting acid-catalyzed reactions
The efficiency of chemical syntheses is determined by the interaction of catalyst and reaction medium. In homogeneous catalysis, high substrate solubility, fast kinetics, high yields and the recyclability of solvent and catalyst are desirable. As acid-catalyzed model reactions, esterifications of various organic acids (e.g. formic, acetic or lactic acid) with short-chain alcohols are investigated, using heteropolyacids (HPA) as catalysts, which are well known and used in the working group in a variety of applications.
Due to water formation during the reaction process, a reaction medium is required that keeps the thermodynamic water activity as low as possible, but at the same time maximizes the activity of the catalyst (proton activity) and the reactants in order to shift the thermodynamic equilibrium to the product side. When using a single solvent, often only one of these properties is achieved, e.g. reducing the water activity while also slowing down reaction kinetics. For this reason, solvent mixtures that deviate from Raoult's law due to strong interactions will be used in SNISMs. These can be, for example, organic solvents in combination with urea, terpenoids or sugars. The influence of SNISMs on the phase behavior, yield and reaction kinetics is not well known so far and will be investigated both experimentally and with a thermodynamic prediction tool.
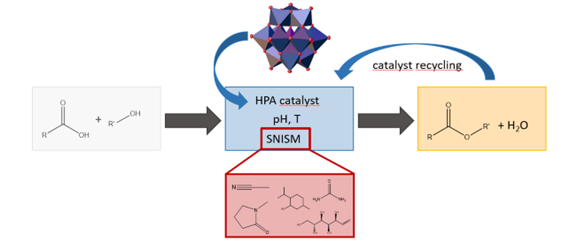
This project is jointly carried out with the working group of PD Dr.-Ing. Christoph Held at the Chair of Thermodynamics at TU Dortmund University. Funded by the German Research Foundation (DFG) under the funding code AOBJ: 699314.
Contact: Lasse Prawitt (lasse.prawitt"AT"uni-hamburg.de)
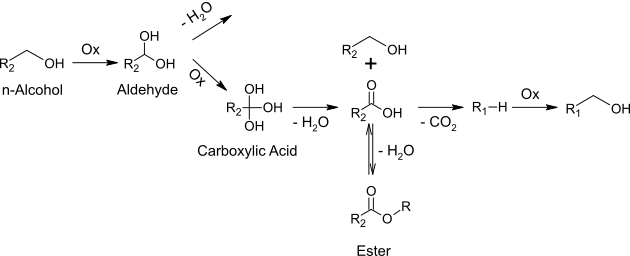
Selective catalytic conversion of humins to low-molecular carboxylic acids using specifically designed polyoxometalates
The worldwide increase in consumption of energy, the growing production of waste and the rising environmental awareness of our society are the driving forces behind the development of sustainable processes. Furthermore, the current dependence of the production of energy and chemicals on fossil raw materials, which are known to be finite, leads to the increasing importance of renewable raw materials such as biomass. These materials offer the potential to grant access to sustainable platform chemicals and secondary energy sources through innovative processes.
Admittedly the number of processes that can convert biomass directly to value-added chemicals are quite low. One of the reasons for this fact is the formation of difficult to process byproducts such as humins (Fig. 1), which arise, for example, in the conversion of cellulose to levulinic acid. The processing of these complex humins represents a substantial challenge for research and development.
The present project deals with the development of a sustainable process for the oxidative conversion of humins to low molecular organic carboxylic acids (formic acid, acetic acid) using homogeneous polyoxometalate catalysts (POM) in the aqueous phase (Fig. 2). Advantages of this process are the mild reaction conditions and the low price of the precious metal free catalysts.
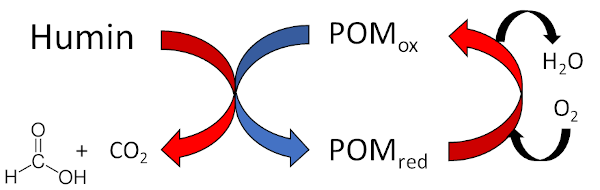
One of the major goals of this project is to gain a fundamental understanding of this completely new valorization method for humins with respect to technical implementation through reaction engineering studies. Special attention is also paid to the synthesis of POM structures with appropriate properties for the oxidation of humins. Additional investigations regarding the isolation of the products and the recycling of the used catalysts will be conducted.
This project is generously funded by the Deutsche Forschungsgemeinschaft (DFG) under funding code AOBJ: 666125 over a period of three years.
contact persons: Andre Wassenberg (Synthese), Tobias Esser (Katalyse)
OxFAMem - Separation and recycling of homogeneous catalysts from process wastewater
OxFA GmbH (client) receives funding from the Bavarian State Ministry of Economic Affairs, Regional Development and Energy (StMWi) for the project "Research into the release and conversion into electricity of biomass-derived hydrogen from bio-formic acid as a storage material".
The Albert research group at the Institute of Technical and Macromolecular Chemistry at the University of Hamburg (UHH) is carrying out research and development work on the separation/recycling of various catalysts from process wastewater for the client as part of a subcontract. The goal of the subcontract in the above-mentioned research project is the separation/reuse of the catalyst or the purification of possible process wastewater using membrane processes.

In close coordination with both partners, possible side streams from oxidation and dehydration are to be evaluated with an eye to further use. For this purpose, the side streams such as process water from oxidation or rectification of the OxFA process will be examined more closely and, if necessary, further treated for a subsequent process.
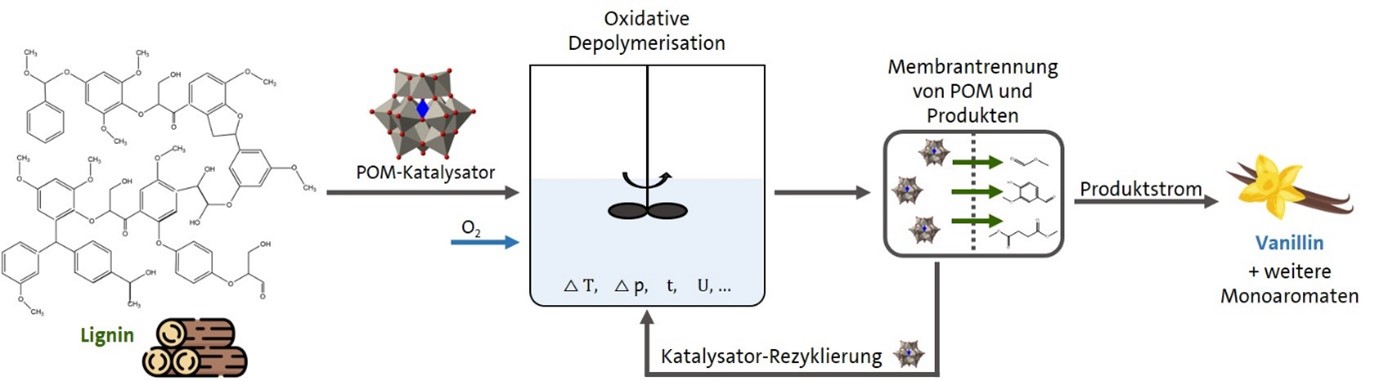
POMLig (Continuous selective oxidative depolymerization of lignin to monoaromatics)
Starting on April 1, 2021, the project "Continuous selective oxidative depolymerization of lignin to valuable monoaromatics using polyoxometalate-based catalysts and just-in-time product removal (POMLig)" will be funded by the Agency for Renewable Resources (Fachagentur Nachwachsende Rohstoffe e.V.). (FNR) with industry participation under the funding code 2219NR439 for three years.
Lignin is a complex, hydrophobic aromatic macromolecule and represents one of the most abundant renewable biobased polymers on earth. Due to its many aromatic groups, lignin is of particular interest for the production of low molecular weight aromatics. However, currently there is no industrial process for selective recovery of the aromatic compounds contained in lignin. Accordingly, low-molecular aromatics are mainly produced petrochemically at the present time. This means that production does not conserve resources and is also directly dependent on the availability of fossil raw materials and the price of crude oil. The renewable biopolymer lignin, on the other hand, is mainly produced as a by-product stream in the paper and pulp industry, as well as in companies that process biogenic fibers. Instead of merely utilizing these by-product streams for energy recovery, as is currently the case, the lignin-containing streams are to be oxidatively depolymerized into low-molecular aromatics as part of the funded project.
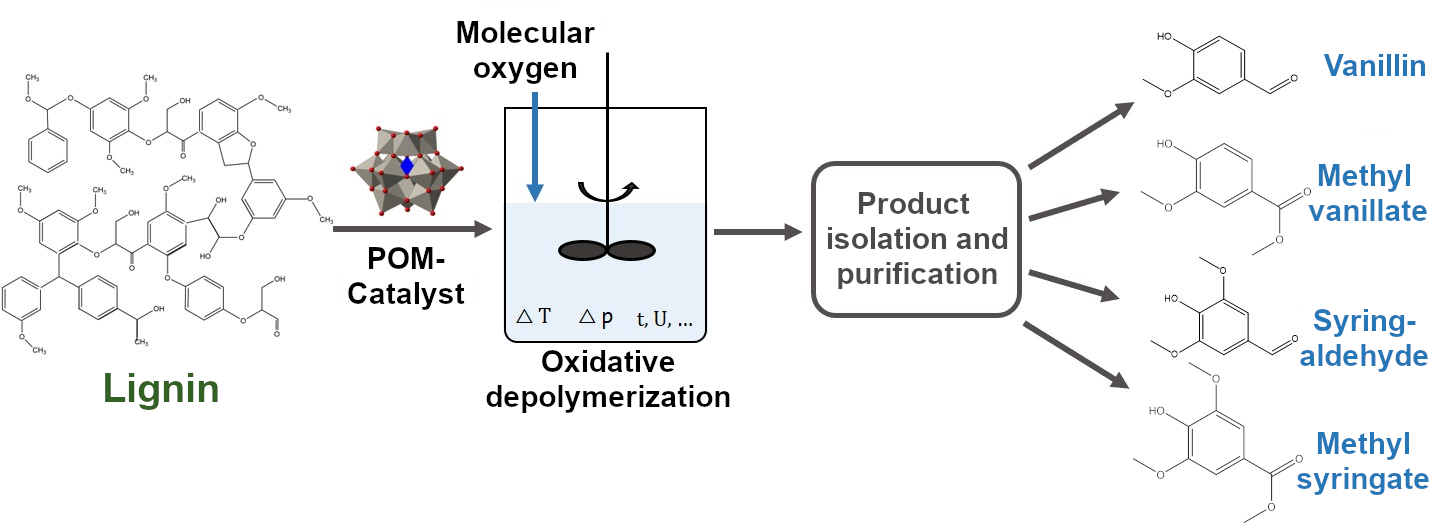
The aim of this project is the efficient and continuous production of monoaromatics, such as vanillin, methyl vanillate, syringaldehyde and methyl syringate from industrial by-product streams containing lignin. In addition to optimizing the catalyst system, the aim is to establish a so-called just-in-time removal of the target products from the reaction medium. This is intended to prevent further undesired subsequent reactions between the target products and the catalyst and thus maximize the yields of the desired products.
The degradation of the lignin-containing by-product streams into valuable monoaromatic materials should thus contribute to the desired independence from fossil raw materials.
Contact: Max Papajewski