Research areas
Research - General information
The main research activities of Rehder’s group have focussed on biological and medicinal aspects of vanadium, (cooperation, in part, with Ebbe Nordlander, Lund University; and Beate Meier, Klinik für Kleintiere, Hannover). Vanadium is a biologically relevant metal, employed by a variety of organisms (Fig. 1): Vanadium is in the active centre of two groups of enzymes, viz. vanadate-dependent haloperoxidases and vanadium-nitrogenases. In addition, vanadium is accumulated by certain life forms such as sea squirts (Ascidiaceae) and Amanita mushrooms, for example the fly agaric. More generally, vanadium appears to be involved in the regulation of phosphate-metabolising enzymes in plants, animals and humans; the insulin-mimetic potential and other medicinal aspects of many vanadium compounds is related to this action.
Vanadium compounds are also widely used as catalysts in oxidation reactions; soluble “vanadium oxides” (polyoxidovanadates) are a more recent development in this field. 51V NMR is a meaningful method for the characterisation of such catalysts. The related giant polyoxidomolybdates have been investigated, inter alia by 7Li and 23Na NMR, in the context of their model character for the cellular cation (counter) transport (cooperation with Achim Müller, University of Bielefeld, and Erhard Haupt, Hamburg). An intriguing aspect in the context of vanadium oxides in catalysis is the occurrence of VO in interstellar and circumstellar gas and dust clouds.
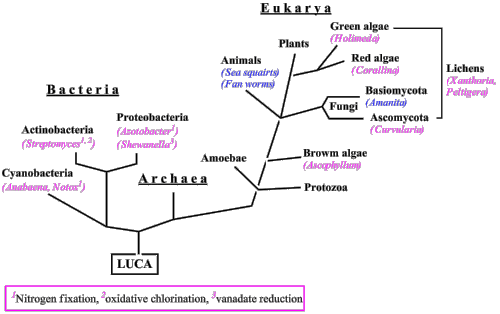
Figure 1. Phylogenetic tree, showing organisms that use vanadium (in red) or accumulate vanadium (in blue). LUCA = last uniform common ancestor.
Research – Specific Research Topics
(1) Vanadium in Biology and Catalysis
Our studies on the biological chemistry of vanadium had been directed towards coordination compounds that model the active sites in vanadate-dependent haloperoxidases (VHPOs, Fig. 2) and nitrogenases, including investigations into the enzyme-substrate interaction. Fig. 2 exemplifies reactions that are catalysed by VHPOs and by structural/functional models of the enzyme’s active centre.
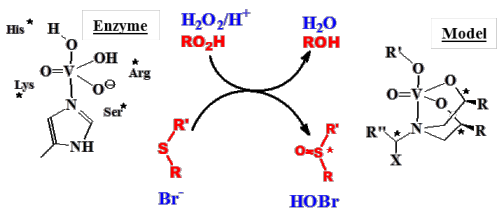
Figure 2. Modelling the enzymatic oxygenation of halide (blue) and sulfide (red). Asterisks indicate chiral centres.
Solution speciation studies of vanadate-ligand and vanadate-peroxide-ligand systems – carried out by multinuclear NMR plus potentiometry (co-operation with L. Pettersson, Umeå) or EPR plus potentiometry (cooperation with E. Garribba, Sassari, and T. Kiss, Szeged), and complemented by investigations of the solid state structures – help to elucidate the structure-function synergism.
Key publications
- The (biological) speciation of vanadate(V) as revealed by 51V NMR – A tribute on Lage Pettersson and his work, D. Rehder, J. Inorg. Biochem. 2015, doi:10.1016/j.jinorgbio.2014.12.014
- Sulfoxigenation catalysed by oxidovanadium complexes, P. Wu, C. Çelik, G. Santoni, J. Dallery and D. Rehder, Eur. J. Inorg. Chem. 2008, 5203-5213.
- Modelling the sulfoxigenation activity of vanadate-dependent peroxidases, P. Wu, G. Santoni, M. Fröba, and D. Rehder, Chemistry and Biodiversity 2008, 5, 1913-1926.
(2) Medicinal Applications of Vanadium
The insulin-mimetic/enhancing behaviour of vanadium compounds, such as their ability to trigger glucose uptake by glucose-metabolising cells, had been investigated in the frame of a Europe-wide COST programme (COST D21-009-01) and in cooperation with the Pharmaceutical University in Kyoto (H. Sakurai). We synthesised vanadium compounds (Fig. 3, top) that minimise toxicity, optimise stability, intestinal absorption and cellular uptake, and enhance the anti-diabetic effects of insulin. – Vanadium complexes containing hydrazone-based ligands have been shown to exhibit antiamoebic activity and are thus potential drugs against amoebiasis (cooperation with M. Maurya, Roorkee, India); Fig. 3, middle and bottom. More generally, transition metal based complexes with macrocyclic ligands have been tested, in cooperation with T. Ghosh Roy and S. Hazari, University of Chittagong, Bangladesh, for their potentiality against parasites that cause tropical diseases.
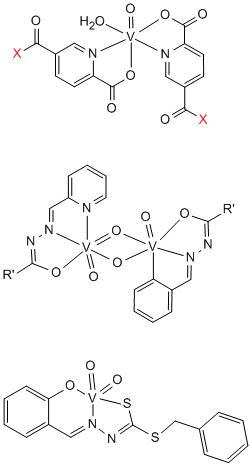
Figure 3. Top: A new family of effective insulin-mimetic vanadium compounds: Bis(1,5-dipicolinato)vanadium(IV). X can be OR (R= alkyl, galactosyl, inositolyl) or NHR (an amino acid residue). Middle and bottom: Compounds which exhibit in vitro anti-amoebic activity; R' = furanoyl or pridyl.
Key publications
- Synthesis and antimicrobial activities of copper(II) complexes of N(4),N(11)-dimethyl (LBZ & LCZ) and N(4)-monomethyl (LCZ1)-3,5,7,7,10,12,14,14-octamethyl-1,4,8,11-tetraazacyclotetradecane. Crystal and molecular structure of [CuLCZ1](ClO4)2, T. Ghosh Roy, S. K. S. Hazari, H. A. Miah, S. K. D. Gupta, P. G. Roy, U. Behrens, D. Rehder, Inorg. Chim. Acta 2014, 415, 124-131.
- Aminoacid-derivatised picolinato-oxidovanadium(IV) complexes: Characterisation, speciation and ex vivo insulin-mimetic potential, H. Esbak, E. A. Enyedy, T. Kiss, Y. Yoshikawa, H. Sakurai, E. Garribba, D. Rehder, J. Inorg. Biochem. 2009, 103, 590-600.
- Bis- and tris(pyridyl)amine-oxidovanadium complexes: Characteristics and insulin-mimetic potential, J. Nilsson, E. Degerman, M. Haukka, G.C. Lisensky, E. Garribba, Y. Yoshikawa, H. Sakurai, E. A. Enyedy, T. Kiss, H. Esbak, D. Rehder, and E. Nordlander, Dalton Trans. 2009, 7902-7911.
(3) Clusters and Cell Models
The functionalisation, "shaping" and stabilisation of polyoxidometalate clusters by their embedment into macro-cycles (such as the cryptand [212]-stabilised decavanadate in Fig. 4, left) allows for the design of “soluble oxides” as homogenous oxidation catalysts. Porous nano-capsules based on polyoxido-molybdates (Fig. 4, right) are models for the cellular transport of alkaline metal ions along ion channels. In cooperation with A. Müller (Bielefeld) and E. Haupt (Hamburg), these phenomena have been investigated by multinuclear NMR (1H, 7Li, 14,15N, 23Na and 39K NMR).
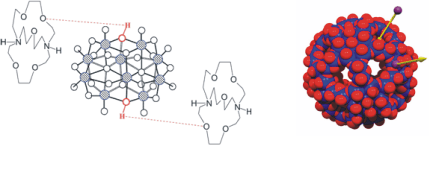
Figure 4. Left: Decavanadate [H2V10O28]4- stabilised by two cryptand cations [C212H2]2+. Right: Featuring Li+ transport through the pores of a Mo132 polyoxidomolybdate.
Key publications
- Guests on Different Internal Capsule Sites Exchange with Each Other and with the Outside, O. Petina, D. Rehder, E.T.K. Haupt, A. Grego, I.A. Weinstock, A. Merca, H. Bögge, J. Szakacs, A. Müller, Angew. Chem. 2011, 123, 430-434; Angew. Chem. Int. Ed. 2011, 50, 410-414.
- A spherical 24 butyrate aggregate with hydrophobic cavity in a capsule with flexible pores: Confinement effects and uptake-release equilibria at higher temperatures, C. Schäffer, H. Bögge, A. Merca, I. A. Weinstock, D. Rehder, E. T. K. Haupt and A. Müller, Angew. Chem. Int. Ed. 2009, 48, 8051-8056.
(4) Interstellar Chemistry
The interstellar formation of water and organics, their delivery to planets and moons, and the implications for Life elsewhere in our Solar System, on rogue planets, and on exoplanets in the habitable zone of their sun, has been documented and discussed in two recent books; see here.
Main funding organisations
German Research Society (DFG), EU (COST), German Academic Exchange Service (DAAD), Free und Hanseatic City of Hamburg.
Cooperations (selection)
Hiromu Sakurai (Suzuka University, Japan), Achim Müller (Universität Bielefeld, Germany), Lage Pettersson † (Umeå Universitetet, Sweden), Ebbe Nordlander (Lund Universitet, Sweden), Saroj Hazari and Tapashi Ghosh Roy (Chittagong University, Bangladesh), Mannar Maurya (Indian Institute of Technology, Roorkee, India), Eugenio Garribba (University of Sassari, Italy), Tamás Kiss (University of Szeged, Hungary), Tatyana Polenova (University of Delaware, Newark, USA), Giulia Licini (Padova University, Italy).
