Two Dimensional Nanosheets
our experts: Thomas Tsangas, Moritz Wehrmeister, Charlotte Ruhmlieb.
Nanosheets are structures that show quantum confinement in just one dimension, which is the thickness. Especially two-dimensional metal-chalcogenide-structures are of great interest due to their unique properties. In addition, they are particularly suitable for structural as well as optoelectronic single-particle investigations. The modification of these nanosheets, e.g., by a cation-exchange or by nanoparticle attachment, results in new unique optical and electronic properties of the material. In our group, copper-sulfide (CuS) nanosheets are synthesized, modified by various wet-chemical methods, and individually investigated regarding their optical and electronic properties.
Synthesis of CuS Nanosheets
For the wet-chemical synthesis of CuS nanosheets, a copper-containing precursor and a sulfur-containing component are continuously mixed at high temperatures in organic solvents, e.g. oleylamine, by using computer-controlled syringe pumps. Due to the crystal structure of the copper-sulfide and the surface-active ligands, two dimensional nanoplatelets or larger nanosheets form.
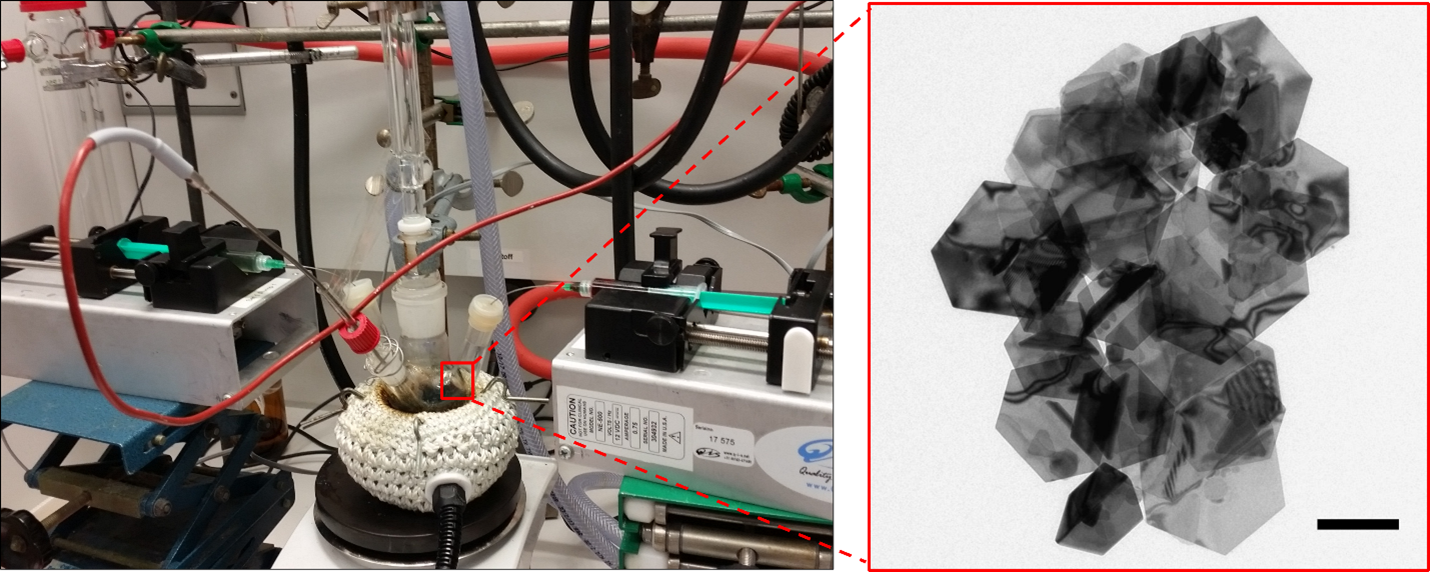
Gold Growth on CuS Nanosheets
Using a synthesis approach developed in this working group, spherical gold nanoparticles can be selectively grown on the side surfaces of the copper-sulfide nanosheets using a reducing agent, a gold precursor compound, and ligands, resulting in defined semiconductor-metal hybrid structures.
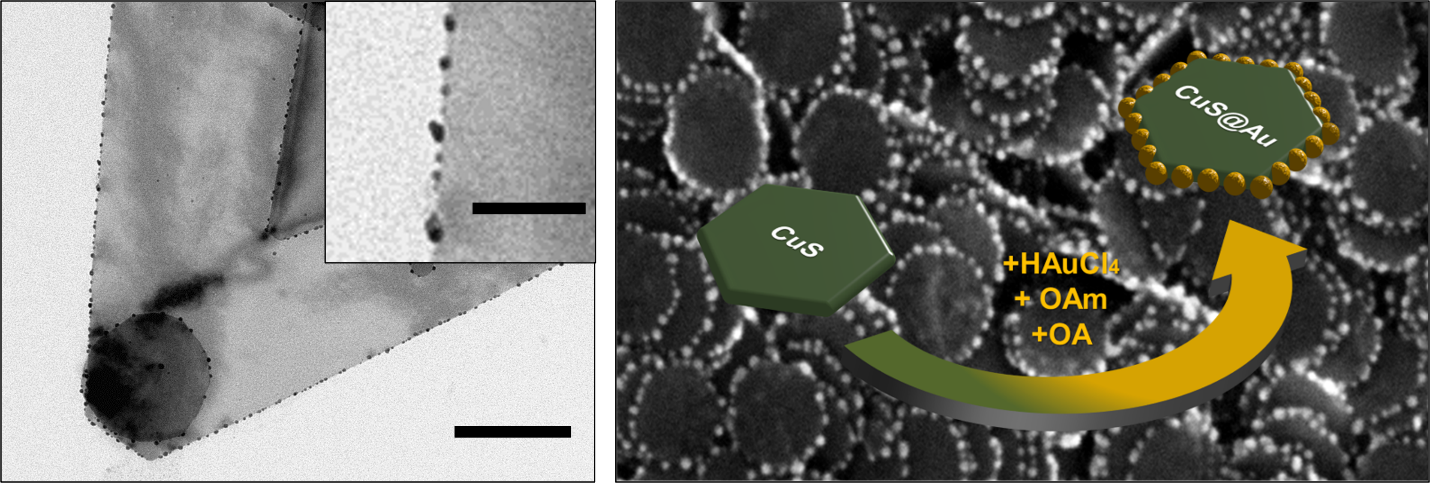
Cation Exchange on CuS Nanosheets
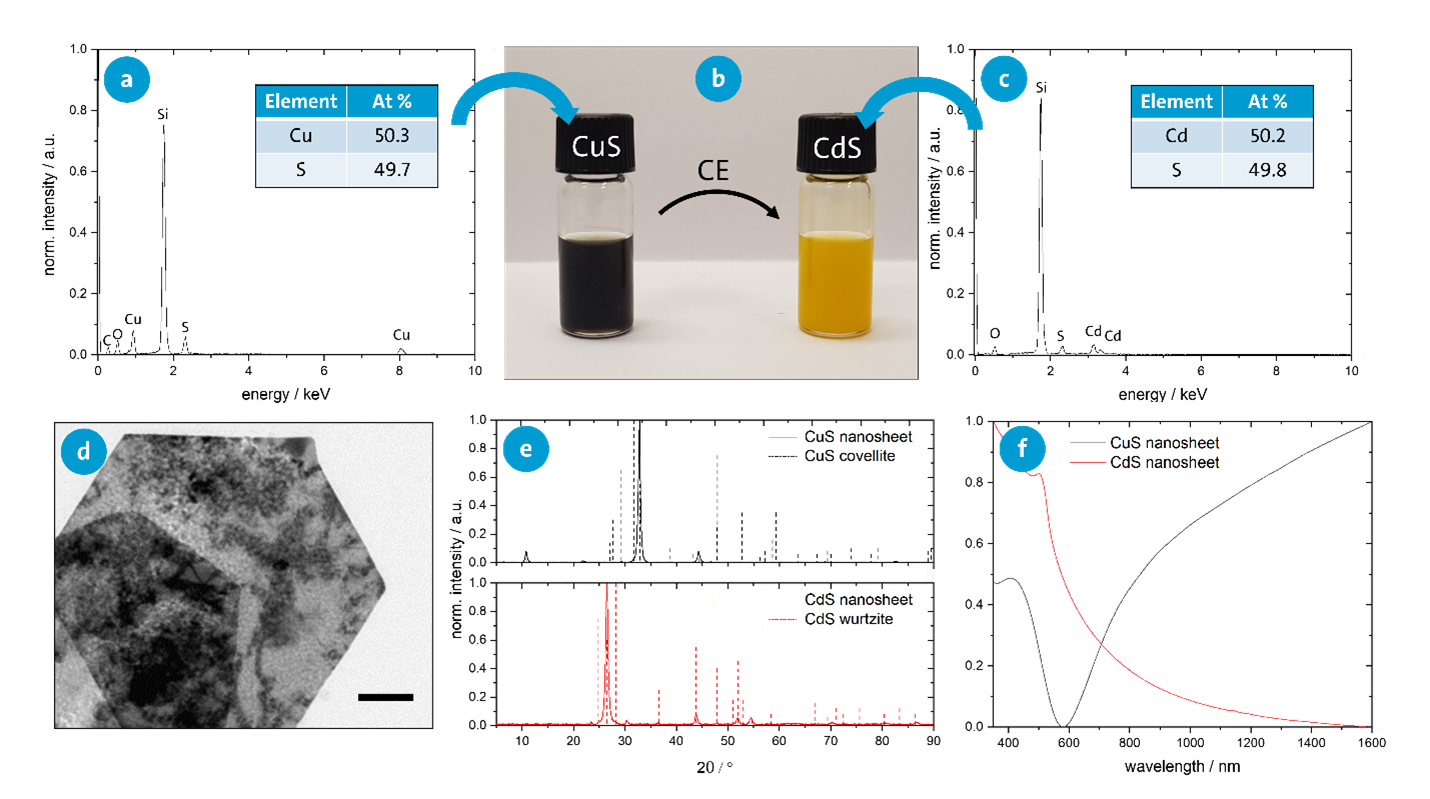
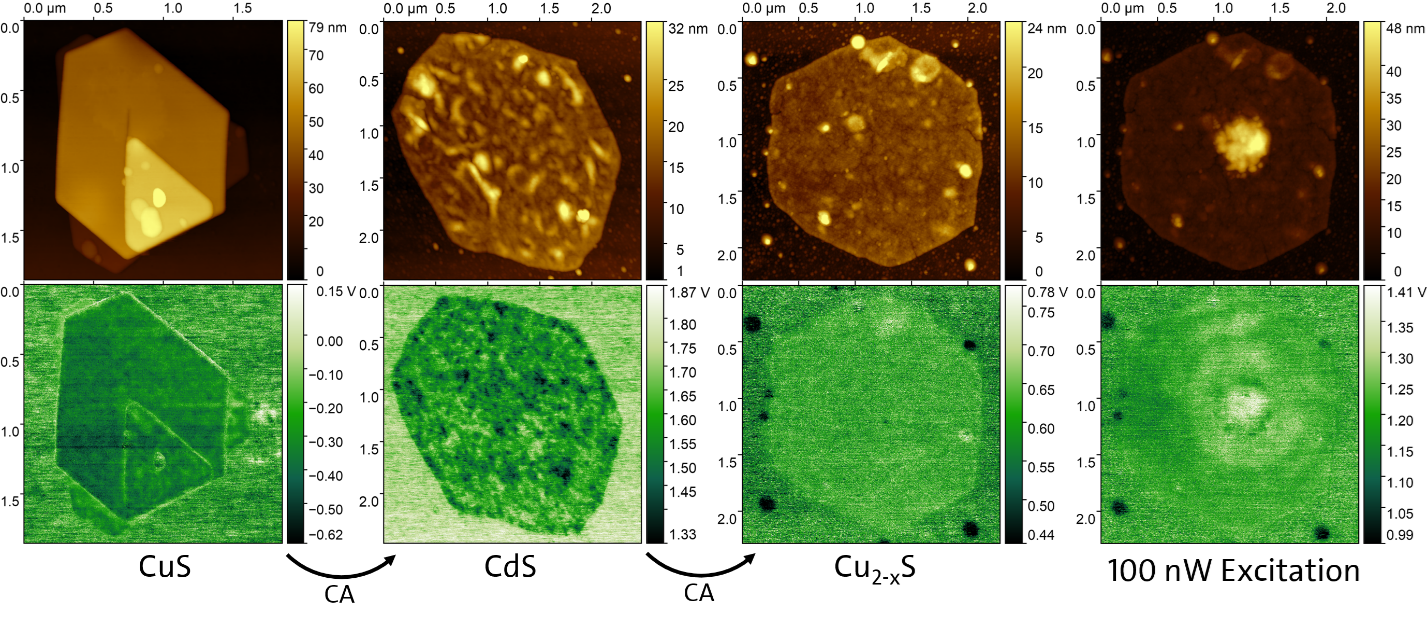
For the modification of the optical and electronic properties of the nanosheets, in addition to a morphological change of the nanostructures, cation exchange (CE) can be performed (e.g., the exchange of copper in CuS nanosheets with cadmium). Various analytical methods can be used to study the modified structural and optical properties.
Analytical Setup
After depositing single nanosheets on a transparent substrate, the movement of charge carriers upon excitation can be analyzed using Kelvin probe force microscopy (KPFM).
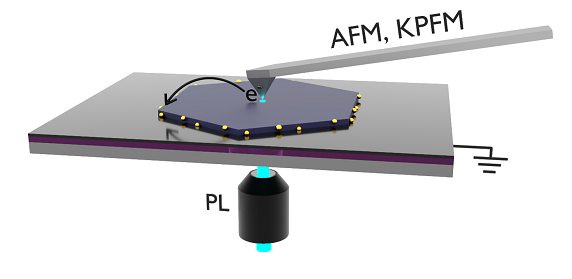
The transparent substrate consists of an insulating layer of silicon dioxide, which is grounded from below by a layer of indium tin oxide (ITO). This allows for optical excitation by a laser from below, while measurements are performed from above using a probing tip.
For semiconductor-metal hybrid structures, it is to be expected that that upon optical excitation of the semiconductor, charge carriers (either the holes or the electrons) move into the metal nanoparticles. Since the work function of both gold and CuS is in the range of 5.1-5.3 eV, it is unclear which kind of charge carrier will move into the metal. Using KPFM, this can be directly observed along with the respective charge-carrier mobilities.
Kelvin Probe Force Microscopy on Nanosheets
The surface potential of nanosheets gives insight about the occurrence of stray charge carriers. It can be measured using KPFM, which is a scanning probe technique. After cation exchange, a change in surface potential (bottom) can be observed. While for CuS the surface potential along the sheet is smooth, for CdS a roughness is observed. Lighter patches indicate the presence of holes while darker patches indicate accumulations of electrons. This indicates that the charge-carrier mobility in CdS is lower than in CuS. Hence, the charge carriers distribute themselves more evenly in CuS. During the excitation of the nanosheet with a laser, an increased potential around the excited position can be seen. This shows that the electrons have a higher mobility than the holes. Optically generated electrons move away from the laser spot leaving behind the holes that can be observed.
Publications
- T. Tsangas, C. Ruhmlieb, S. Hentschel, H. Noei, A. Stierle, T. Kipp, A. Mews, Controlled Growth of Gold Nanoparticles on Covellite Copper Sulfide Nanoplatelets for the Formation of Plate–Satellite Hybrid Structures. Chem. Mater. 2022, 34 (3), 1157–1166 (https://doi.org/10.1021/acs.chemmater.1c03631).
- M.M. Kobylinski, C. Ruhmlieb, A. Kornowski, A. Mews, Hexagonally Shaped Two-Dimensional Tin(II)Sulfide Nanosheets: Growth Model and Controlled Structure Formation. J. Phys. Chem. C 2018, 122 (10), 5784–5795 (https://doi.org/10.1021/acs.jpcc.7b12567).