Specific Plant Lectins
One research focus of the group is the detailed analysis of selected plant proteins, here particular the X-ray analysis of ribosome inactivating proteins (RIPs) of type I and II. We have analysed the native and inhibited structure of mistletoe lectin I from Viscum album. Viscum album is the "evergreen plant" growing in symbiosis with trees. Viscum album is also known as medicinal herb for almost 2000 years. The protein Mistletoe-lectin I (ML-I) is today the main component and is used in the alternative cancer therapy. Mistletoe-lectin I is a 63 kDa heterodimeric protein consisting of two subunits: a toxic A unit and a lectin-binding B chain. The toxic subunit is able to inactivate very effective eukaryotic ribosomes by the specific cleavage of ribosomal RNA leading to immediate cell death. Therefore RIPs are today also classified as “Biowappens” and distinct research programs are focussed to identify RIP suspensions and to identify effective inhibitors.
The second subunit of RIPs is responsible for recognising and targeting the cell membrane and the subsequent cell-uptake of the toxic subunit. However, since the detailed mode of action for many RIPs is still unknown till now the enlightenment of the three-dimensional structures will provide further insights about the structure-function relationship.
The second subunit of RIPs is responsible for recognising and targeting the cell membrane and the subsequent cell-uptake of the toxic subunit. However, since the detailed mode of action for many RIPs is still unknown till now the enlightenment of the three-dimensional structures will provide further insights about the structure-function relationship.
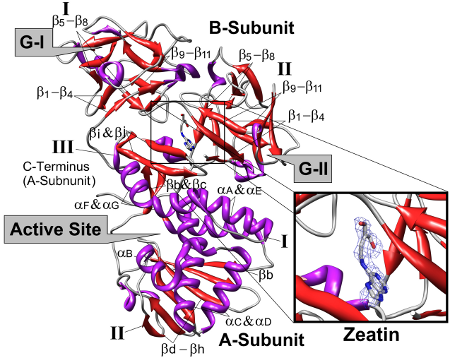
Three-dimensional structure of Viscum album mistletoe lectin I in complex with a phytohormone.
Selected Publications
- A. Meyer, W. Rypniewski, M. Szymanski, W. Voelter, J. Barciszewski, and Ch. Betzel. "Structure of mistletoe lectin I from Viscum album in complex with the phytohormone zeatin". Biochim. Biophys. Acta 1784, 1590-1595 (2008).
- A. Meyer, W. Rypniewski, L. Celewicz, V. Erdmann, W. Voelter, T. P. Singh, N. Genov, J. Barciszweski and Ch. Betzel. “The Mistletoe Lectin I – Phloretamide Structure reveals a New Function of Plant Lectins”. Biophys. Biochem. Res. Commun. 364, 195-200 (2007).
- R. Krauspenhaar, W. Rypniewski, N. Kalkura, K. Moore, L. DeLucas, St. Stoeva, A. W. Voelter and Ch. Betzel "Crystallisation under Mircrogravity of Misteltoe Lectin I from Viscum album in Complex with Adenine Monophosphate and the Structure at 1.9Å Resolution" Acta Cryst. D58, 1704-1707 (2002).
