Research
Bioorganometallic Chemistry and Tumor Targeting
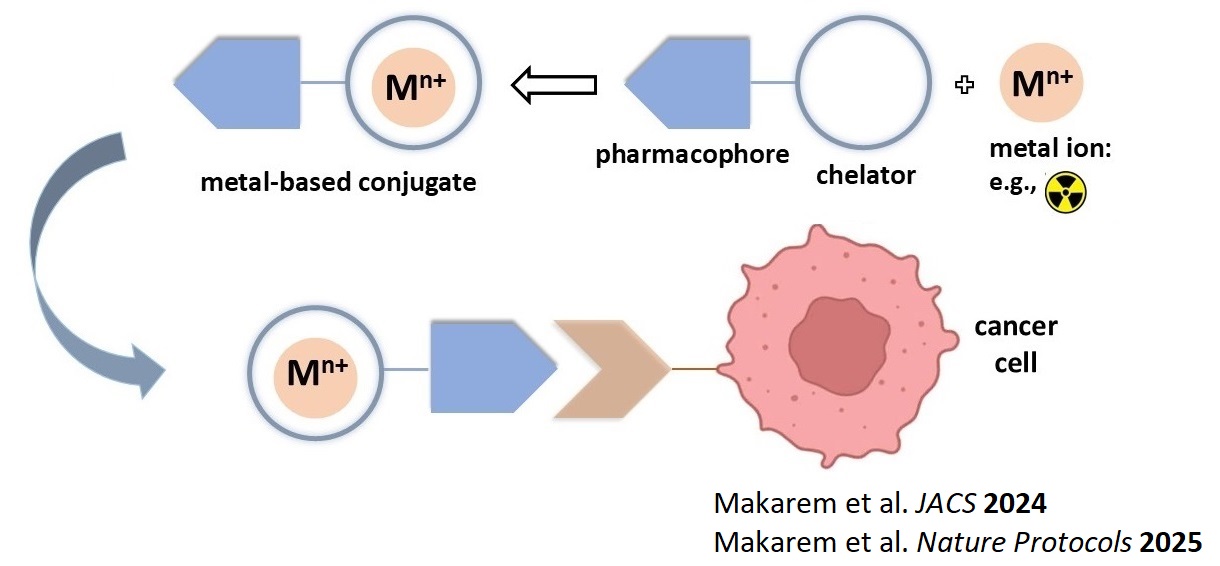
In our fundamental research we investigate metal-based conjugates for use in tumor
targeting and cancer theranostics (e.g., PET imaging, targeted therapy). Metal-based
conjugates are modified bioactive molecules (peptides, proteins, antibodies, etc.) bearing a
theranostic metal/radiometal ion and are currently of high interest in radiopharmaceutical
drug discovery and nuclear medicine. They are structurally composed of three parts: a
bioactive targeting molecule or pharmacophore, a metal/radiometal payload, and a
bifunctional complexing ligand (chelator) which is a small organic molecule linking the metal
payload to the pharmacophore. The research in Makarem Lab particularly focuses on new
bifunctional chelators.
This class of complexing ligands can coordinate metal ions and simultaneously bind to
bioactive vectors; they are duly called “bifunctional” chelators. For clinical applications, the chelate cage must be strong enough to safely deliver its metal/radiometal payload to tumor cells. Additionally, the linkage between the chelator backbone and the pharmacophore must remain stable during the biological circulation in patient’s body. Hence, chelators designed for tumor targeting must be assessed from different perspectives, including coordination properties, conjugation behavior, and biostability. To achieve this goal, we primarily investigate non-radioactive (“cold”) models, while successful results will be further developed using radioactive approaches.
In this context our research is divided into 3 steps.
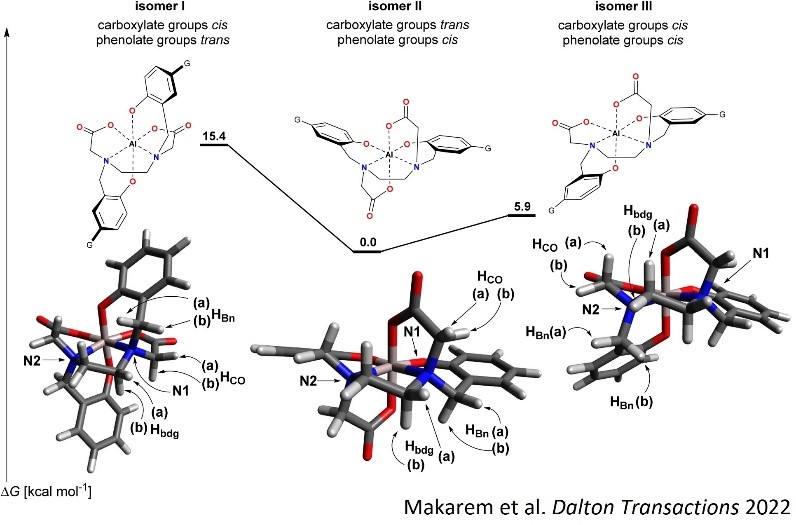
1- Design and synthesis:
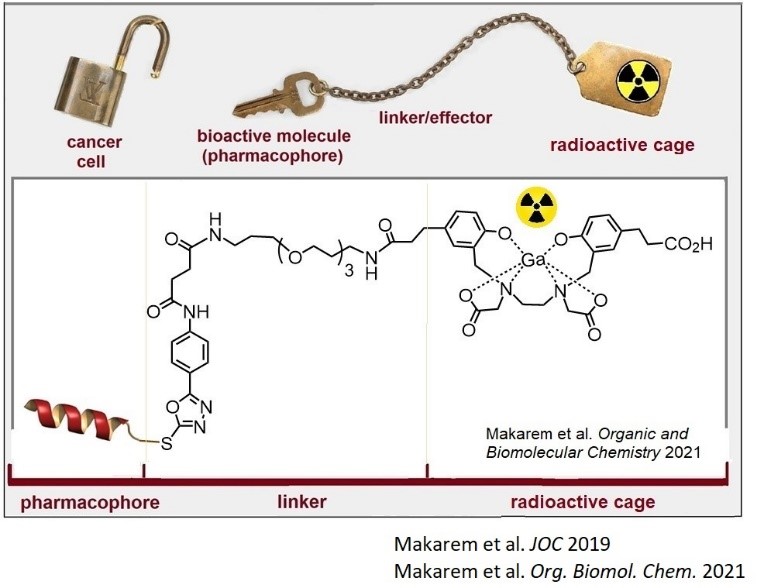
2- Molecular studies (complex chemistry, bioconjugation methodologies, stability tests, etc.):
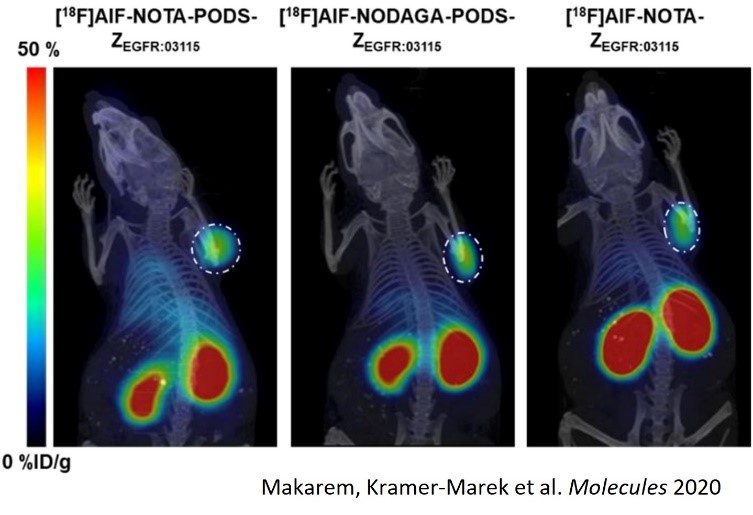
3- Applications in radiopharmaceutical syntheses and tumor targeting (collaboration with partners)