Water - An unusual compound

Water is the basis of all life, our earth's surface is covered by 71% of it, a large part of it is salt water in the seas and oceans. But also the human consists of more than half (depending on age about 50-70 %) of water. In nature it is seldom pure, but usually contains salts, organic compounds or even gases. In its pure form it is colourless, odourless and tasteless.
Water is also the only chemical compound on earth that occurs naturally as a liquid ("water"), as a solid ("ice") and as a gas ("water vapour") (Figure 1). Our climate and weather are largely determined by these three states of matter. The triple point of water, at which the three phases of pure liquid water, pure solid water and water vapour are in equilibrium (Figure 2), is 273.16 K and 0.0061 bar and has long served as a fixed point on the international temperature scale.
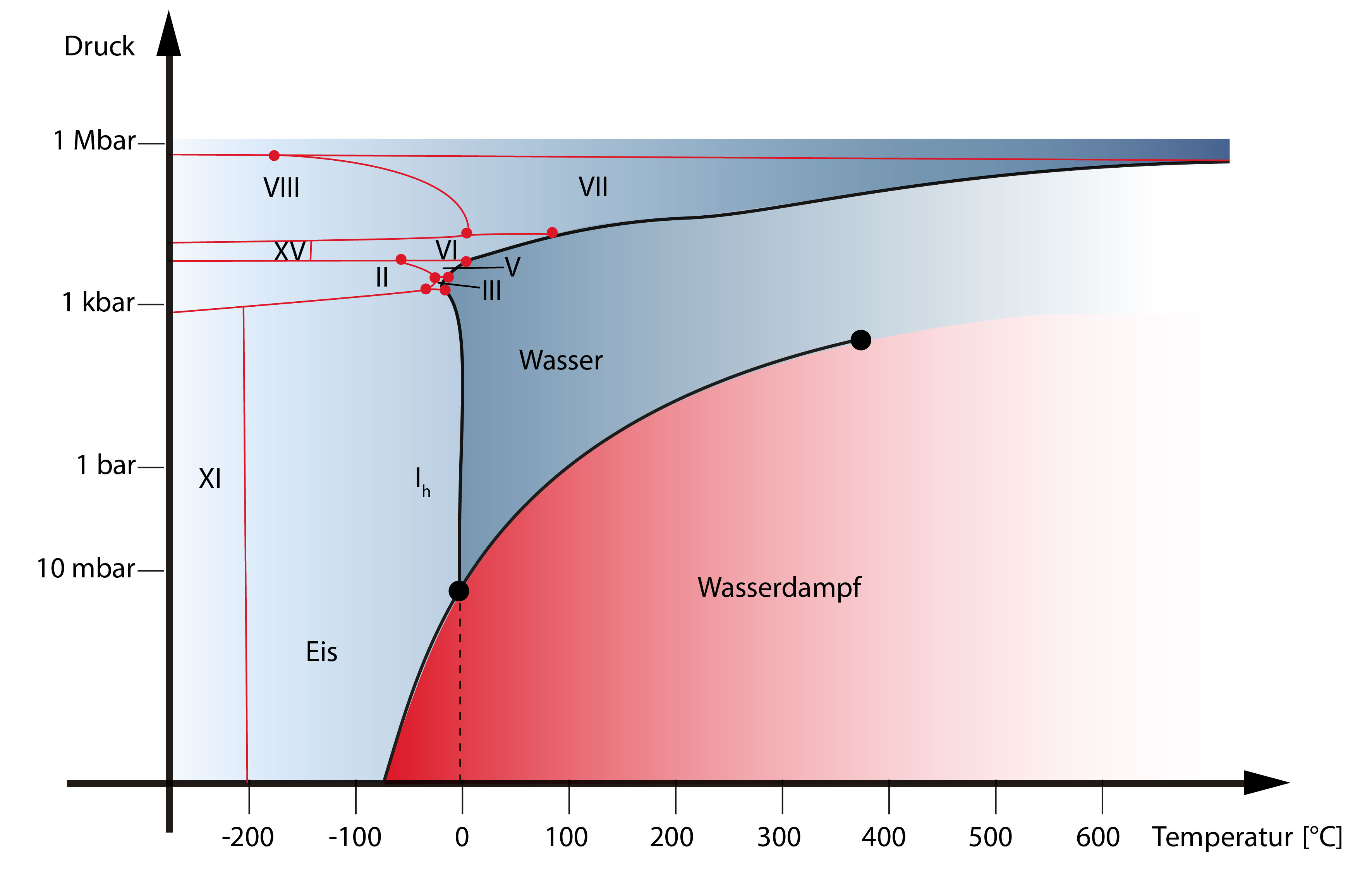
Water may be one of the best known and most important substances on the planet, but it is certainly not ordinary and its properties are still the subject of intensive research.
Even as a volume system, water has special properties compared to other fluids, such as the famous density anomaly. While most fluids contract on cooling, water expands again (at normal pressure) below 4 °C, the density decreases. Compared to its size, the boiling point of water (100 °C) is also much higher than one would expect.
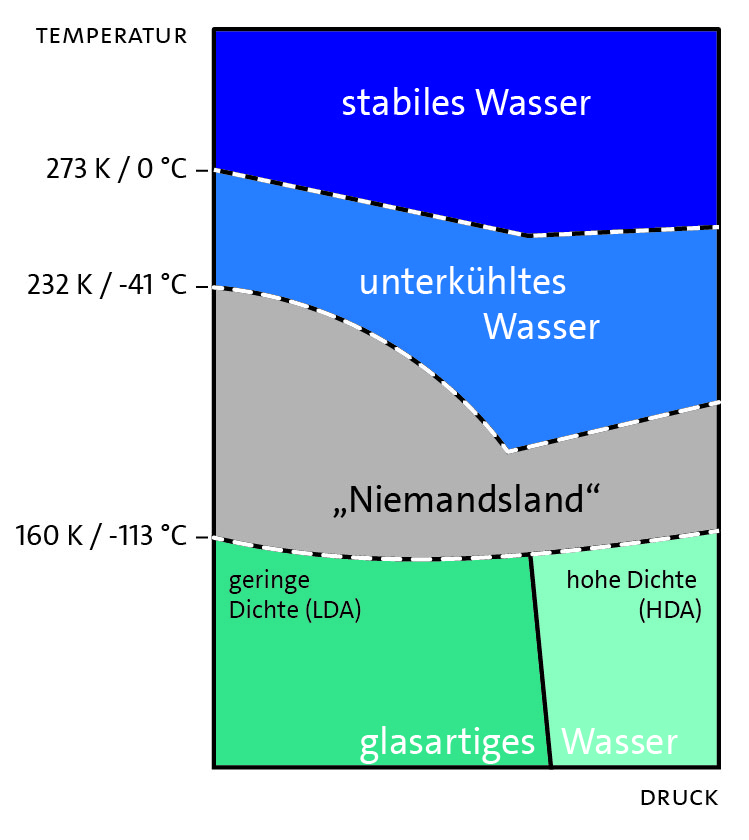
Water has numerous other anomalies (about 60 are currently known), many of which become dominant especially in the supercooled state (depending on the pressure in the temperature range between about 0 to -100 °C and under the condition that no crystallisation nuclei are present, otherwise ice is formed spontaneously). If the temperature is lowered even further, the phase diagram (Figure 3) initially shows the so-called "no man's land", metastable liquid water, which is difficult to investigate experimentally, since water crystallizes spontaneously here, even without a nucleus. If one advances to even lower temperatures (approximately from -113 °C), it is possible, depending on the pressure, to achieve glassy, low or high density amorphous ice forms.
The core of all these phenomena, the properties of water in general, are, in addition to the dipole moment of the molecule, the so-called hydrogen bonds, which are an intermolecular interaction between a hydrogen atom of one water molecule with the oxygen atom of an adjacent water molecule. A single water molecule can form up to four hydrogen bonds in tetrahedral coordination (Figure 4 (a)) and thus has the ability to form three-dimensional networks.
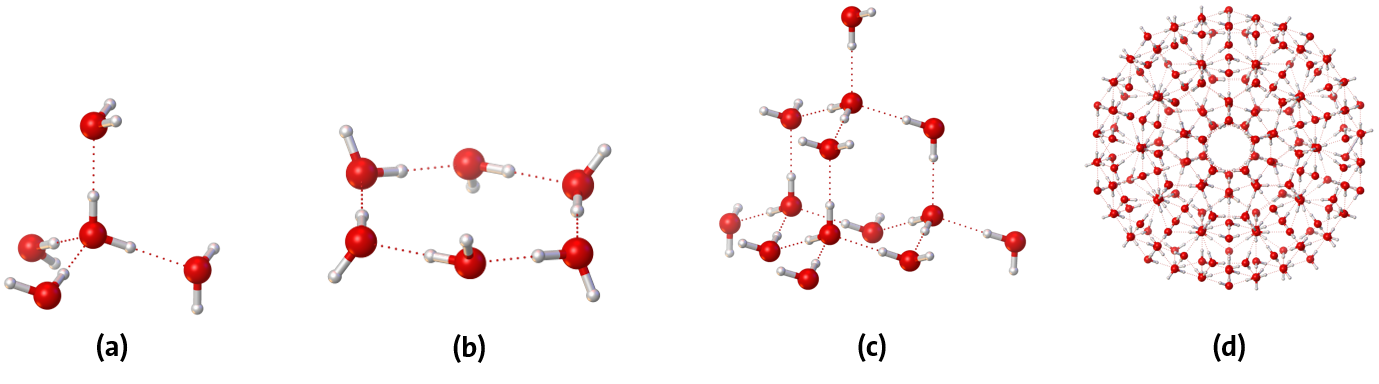

Depending on the pressure or temperature range (and the associated state of matter), numerous two- and three-dimensional structures, clusters of water molecules of different sizes have been detected. In the gas phase, cages, rings and prisms are the most common. Ice shows a pronounced polymorphism, depending on pressure and temperature, 17 different crystalline, plus the two amorphous forms (already mentioned above) are known so far. A generally known modification is ice Ih, e.g. in the form of snowflakes, the hexagonal symmetry of the crystal structure is reflected here in the outer, hexagonal shape of the ice crystals (Figure 5).
In liquid water, in simplified terms, there is a space-filling network of water molecules that strives to form the energetically preferred, tetrahedrally arranged structure. These typical clusters are tetrahedral (H2O)14 clusters, which then span even larger units such as icosahedral (H2O)280 clusters (Figure 4, (c) and (d)). However, this is always a dynamic network, the hydrogen bonds are constantly being broken and new ones are being formed. A hydrogen bridge only exists for a few picoseconds on average, whereas the structural dynamics within the hydrogen bridge network even reaches the time scale of a few tenths of picoseconds.
However, water is not only present freely and in larger volumes, but often also in a spatially limited form, in nanoscale, pore-like geometries.
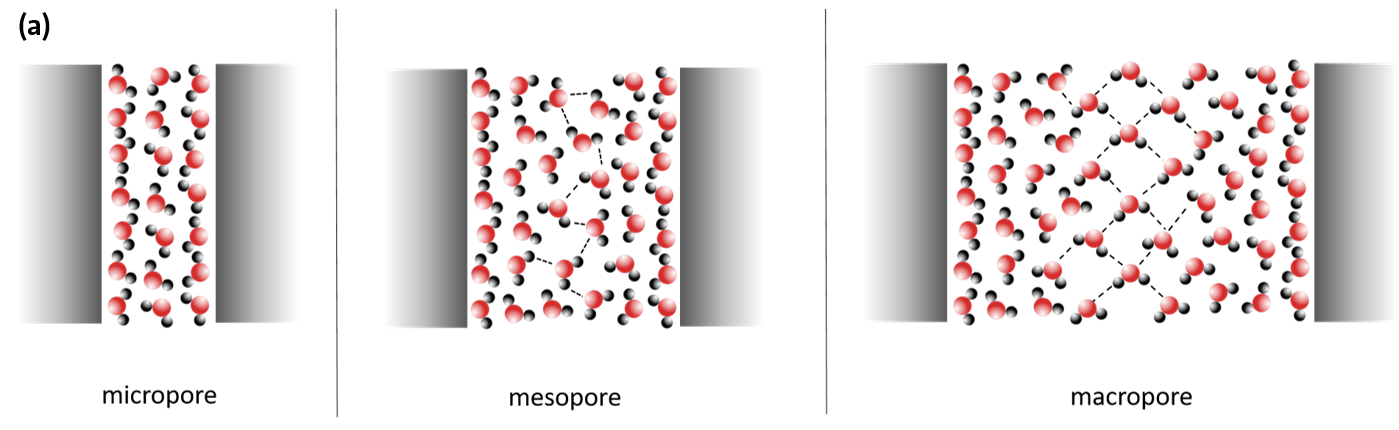
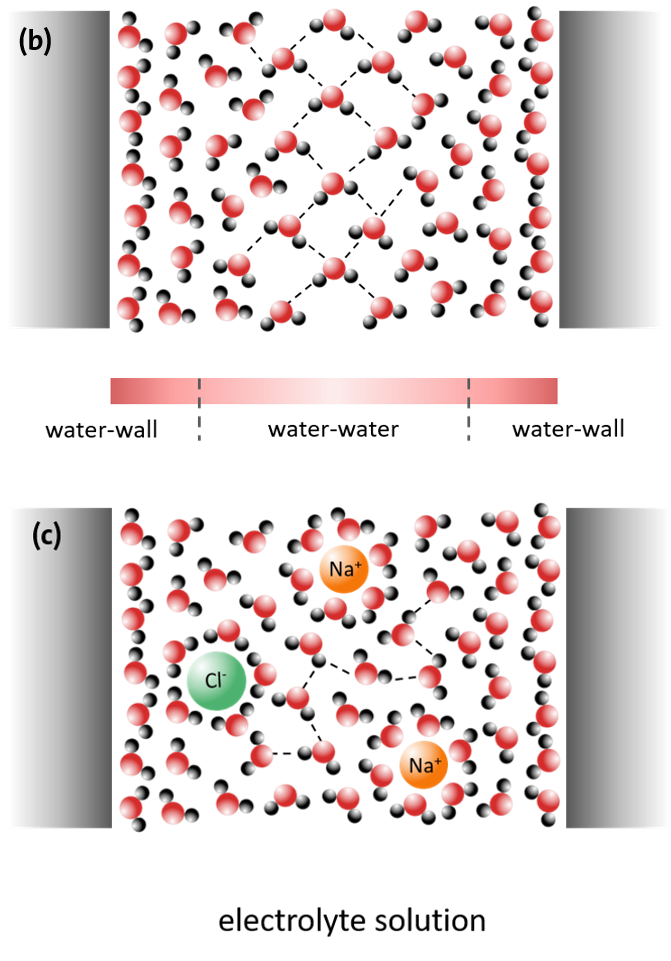
The properties of water are then strongly determined by the spatial extent of the geometric restriction on the one hand and by the locally existing interactions with the surface of the restriction, e.g. a pore wall, on the other hand. The so-called confinement effect occurs at pore diameters of less than 10 nm. Depending on the type of restriction, the interaction with the pore surface and any substances dissolved in the water (electrolytes), the physical and chemical properties of the water are then greatly altered (Figure 6).
This behaviour of the water, which is significantly different from that of the bulk[MF1] system, plays an important role in many biological processes, such as protein folding, in lab-on-a-chip systems, in the solubility of gases or heterogeneous catalysis. Furthermore, the crystallization of ice or dissolved salts under limited geometrical conditions is one of the most important damage processes in natural rock weathering or in various building materials (historical and modern buildings and construction structures such as bridges and tunnels). In addition, the phase equilibria of water and aqueous electrolyte solutions determine the range of existence of liquid water under extreme climatic conditions, for example on Mars. Since liquid water is considered a basic prerequisite for the development of life forms, knowledge of such equilibria is of great importance.
Despite the enormous relevance of water and aqueous solutions, little is known about these underlying processes and the influence of limited geometries on the thermodynamics and structural dynamics of water/hydrogen bonds. This is particularly surprising, since there are already many investigations in this respect for simple molecular fluids, such as linear hydrocarbons, and molecular dynamics studies, among other things, indicate significant changes in the electrical polarizability of water in interface-determined geometries, especially in nanopores. The research group presented here will try to answer some of the open questions regarding the properties of water in nanopores. Details of the specific questions and subprojects can be found here.
