Research
Protein-based nanomaterials
The Beck group works in the field of protein-based nanomaterials. We use protein containers to assemble inorganic nanoparticles into highly structured materials. A range of techniques is employed: protein design using computational tools, protein production and purification, nanoparticle synthesis and nanoparticle assembly with crystallization methods. Characterization of the materials is carried out with X-ray crystallography, electron microscopy and small angle X-ray scattering.
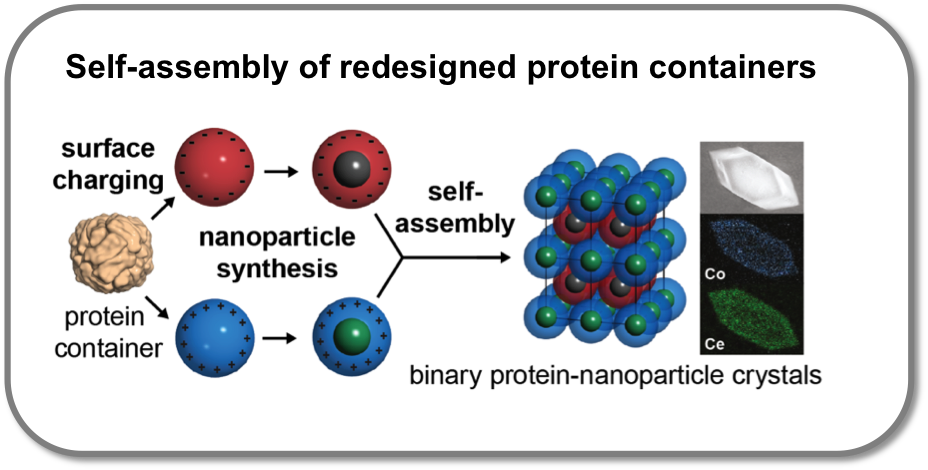
Self-assembly of redesigned protein containers
Self-organization is a key tool for the construction of functional nanomaterials. We have recently established a novel method for the self-organization of biomolecular building blocks and nanoparticles. Here, protein containers, engineered with opposite surface charge, are used as an atomically precise ligand shell for the assembly of inorganic nanoparticles. The assembly of these protein-nanoparticle composites yields highly ordered nanoparticle superlattices with unprecedented precision. The structure of the protein scaffold can be tuned with external stimuli such as metal ion concentration. Along these lines, the protein containers used as a scaffold offer a viable route towards renewable materials.
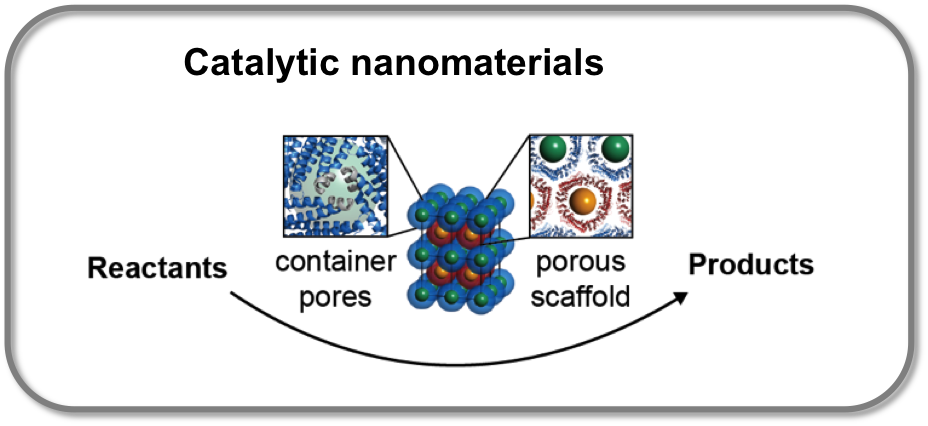
Catalytic nanomaterials
The cavity of the protein container can be filled with metal oxide nanoparticle, prior to assembly of the protein containers. The crystallization of oppositely charged protein containers with nanoparticle cargo yields highly ordered nanoparticle superlattices as free-standing crystals, with up to a few hundred micrometers in size. Moreover, the protein matrix can be stabilized by fixation of the crystals with glutaraldehyde without changing the original crystal structure. The application potential of these biohybrid materials in catalysis was investigated. Unitary cerium oxide nanoparticle superlattices show oxidase-like and peroxidase-like activity. The combination of two different nanoparticles in the protein scaffold does not influence the activity of the single nanoparticle type.
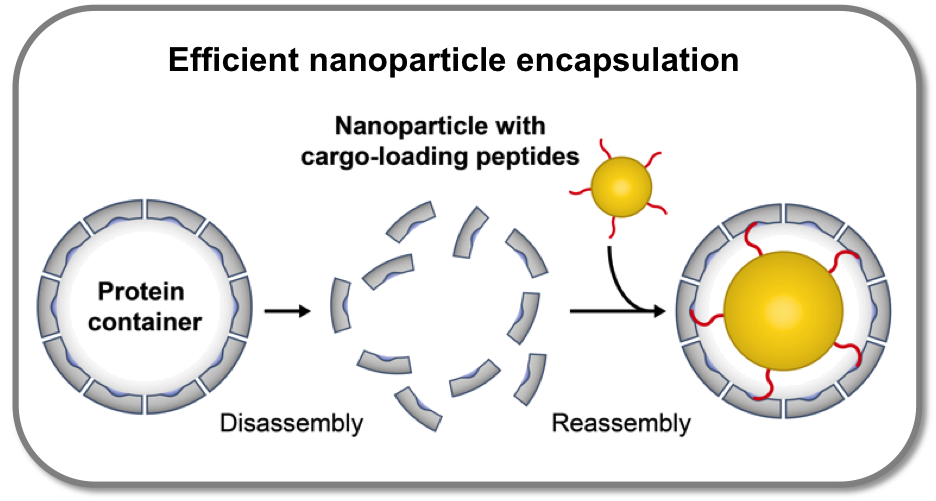
Efficient nanoparticle encapsulation
We demonstrated that the highly specific cargo-loading mechanism of the bacterial nanocompartment encapsulin can be employed for encapsulation of artificial cargo like inorganic nanoparticles. For this purpose, container-filling gold nanoparticles were decorated with a small number of encapsulin cargo-loading peptides. By lock-and-key interaction between the peptides and the peptide-binding pockets on the inner container surface, the nanoparticles are encapsulated into encapsulin with extremely high efficiency. Due to its rigid structure, encapsulin does not adapt to the size of the nanoparticle cargo. Most notably, peptide binding is independent from external factors such as ionic strength. Cargo-loading peptides may serve as generally applicable tool for efficient and specific encapsulation of cargo molecules into a proteinaceous compartment.
Previous work: Protein structure determination with halogenated ligands
