Publication of the month: A Nanobody Against Herpes (RG Prof. Grünewald)
26 September 2025

Photo: Benjamin Vollmer / Centre for Structural Systems Biology, Carmen Rotte / Max Planck Institute for Multidisciplinary Sciences
Herpesviruses infect over 40 million people annually, posing severe risks to newborns and those with weakened immune systems. Researchers from Hamburg and Göttingen have developed a mini-antibody (nanobody) that neutralises glycoprotein B (gB), a key protein for herpesvirus infection. Published in Nature, this breakthrough could enable new therapies to treat and prevent severe herpesvirus infections.
It is here to stay: Once infected, the herpesvirus remains in the body for life, lying dormant in nerve cells and reactivating when the immune system is weakened or stressed. Around 60 percent of people carry Herpes simplex virus 1 (HSV-1), typically causing cold sores, while nearly 20 percent have genital herpes, usually caused by HSV-2. What is primarily painful and unpleasant for otherwise healthy individuals can have drastic, sometimes fatal consequences for people with pre-existing conditions. Severe cases can affect the central nervous system. Newborns are particularly at risk: If the mother has an active herpes infection, the child can easily become infected during birth. This neonatal herpes often results in permanent neurological damage and can even be fatal for the child. Drugs currently available only target active infections and cannot be used prophylactically or in the case of a latent, non-active infection.
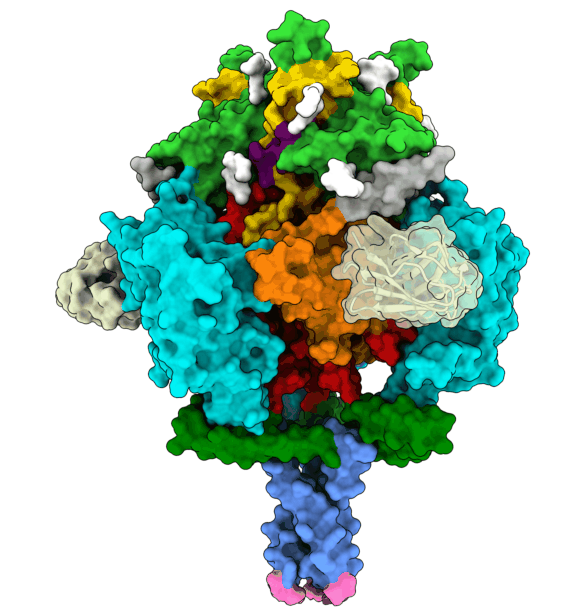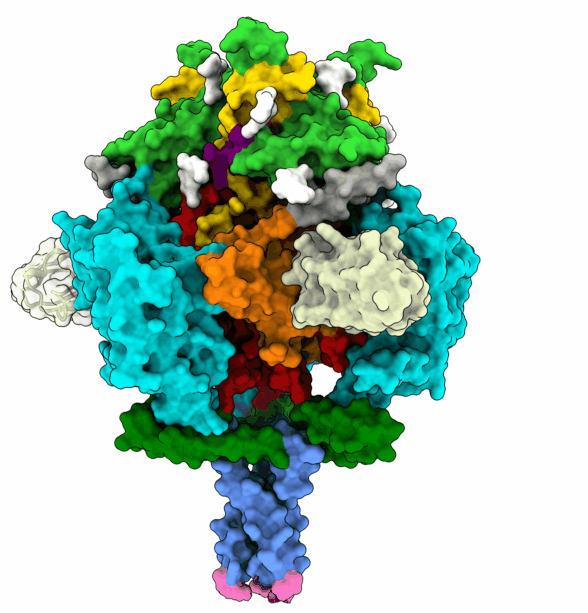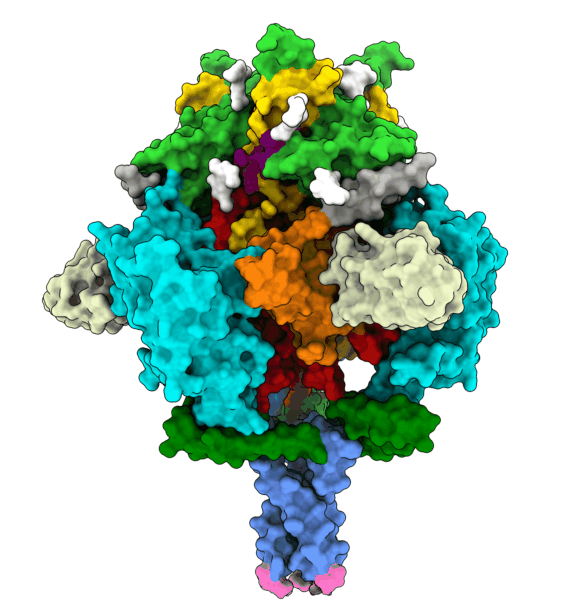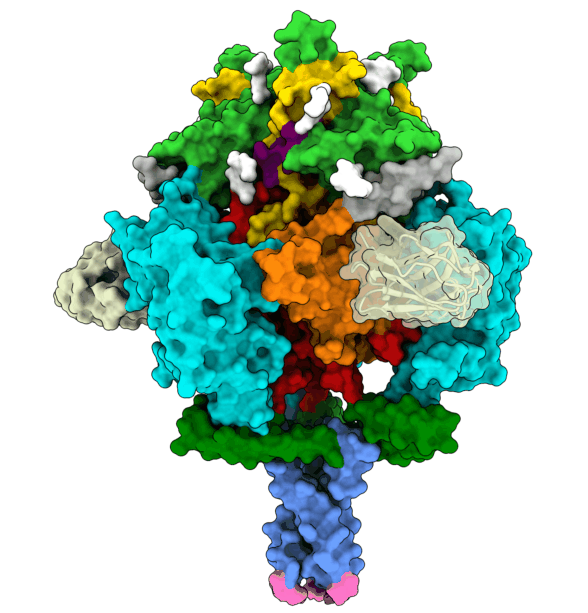
To infect a cell, the herpesvirus first attaches to the cell membrane, then fuses its own membrane with that of the host to release its genetic material and replicate. This fusion is driven by glycoprotein B (gB), which changes its shape to enable this process. The protein is ‘energy-charged’ and uses this energy to fuse the virus envelope with the cell membrane. Researchers at the University of Hamburg (UHH), the Leibniz Institute of Virology (LIV), and the University Medical Center Hamburg-Eppendorf (UKE) based at the Centre for Structural Systems Biology (CSSB) in Hamburg, and the Max Planck Institute (MPI) for Multidisciplinary Sciences in Göttingen have stabilised the gB complex and structurally resolved in its previously elusive, fusion-ready form using cryogenic electron microscopy (cryo-EM). The Göttingen team isolated a mini-antibody, known as a nanobody, from an alpaca that was immunised with the gB protein. From around a billion different nanobodies the gB-specific ones were isolated and tested for their antiviral activity to find one with the potential to neutralise HSV-1 and HSV-2. The nanobody binds to the fusion-ready form of gB and prevents it from performing the conformational movements and releasing the energy required for fusion. In Hamburg, the team succeeded in elucidating the 3D structure of gB bound by the nanobody. This structure, solved using advanced computational modelling and validation tools, revealed insights into critical positions in gB, thereby deciphering the neutralisation mechanism.

The teams’ findings promise a new approach for treating and preventing herpes infections. The found nanobody could both supplement existing treatments and offer protection to people that are at risk due to weak immune systems or prone to recurrent infections, such as newborns, HIV-infected individuals, and patients with cancer, autoimmune diseases, or an upcoming organ transplant. A patent application to further develop the nanobodies for clinical use and to attract industry partners has already been filed.
Original Publication:
Vollmer B, Ebel H, Rees R, Nentwig J, Mulvaney T, Schünemann J, Krull J, Topf M, Görlich D, Grünewald K (2025) A nanobody specific to prefusion glycoprotein B neutralises HSV-1 and -2.
https://doi.org/10.1038/s41586-025-09438-5
Further informations:
https://www.cssb-hamburg.de/news_amp_events/articles/2025/a_nanobody_against_herpes/index_eng.html
https://www.leibniz-liv.de/en/news/news-and-press/a-nanobody-against-herpes
https://www.mpinat.mpg.de/5095326/pr_2515