
A8: The cycloSal concept - an efficient intracellular delivery system for mannose-1-phosphate and a new approach for the synthesis of nucleoside diphosphate sugars (NDP-sugars) -
The cycloSal-concept of C. MEIER is based on the differential stabilities of phenyl, benzyl and alkyl phosphate esters. These allow chemical discrimination between the various phosphate ester bonds and the release of the monophosphate in a coupled hydrolysis (tandem reaction) (Figure I). The cycloSal concept is a well established system for the delivery of nucleoside monophosphates.
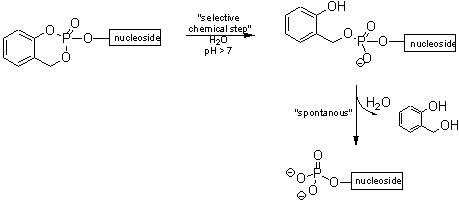
Figure I : Selective delivery of nucleoside monophosphate by a tandem reaction
The prodrug concept of C. MEIER will be applied for the first time to mannose-1-monophosphate. One potential therapeutical application is the hereditary metabolic disease Congenital Disorder of Glycosylation (CDG), in which a genetic defect leads to the inavailability of phosphomannomutase 2 (PMM 2), which hinders the conversion of mannose-6-phosphate to mannose-1-phosphate.
The cycloSal-mannose-1-phosphates will be prepared using the established method for synthesis of cycloSaligenyl-nucleoside monophosphates via cyclosaligenyl chloro-phosphite and subsequent oxidation. (Figure II)
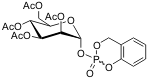
Figure II: acetylated cycloSal-mannose-1-phosphate
Conclusions regarding the hydrolysis mechanism will be drawn based on the chemical stability determined by HPLC, and the distribution and identification of the hydrolysis products using 31P-NMR spectroscopy and ESI-MS/MS investigation.
In addition, the biological activity will be evaluated in vitro on PMM 2-deficient fibroblasts.
A new challenge for the cycloSal concept is the synthesis of nucleoside diphosphate sugars. These are important building blocks in the biosynthesis of oligosaccharides. For this reason a synthesis approach is of essential importance e.g. for the investigation of the biosynthesis of lipopolysaccharides (LPS). Existing methods for the synthesis of nucleoside diphosphate sugars seldom exceeds yield of 30-40 %.
In the new route the cycloSal phosphate triester plays a supporting role as an activated phosphate acceptor. As mentioned above the cycloSal concept is based on a chemically induced cascade mechanism. The initial step is a nucleophilic attack by water or hydroxide ion at the phosphorous atom.
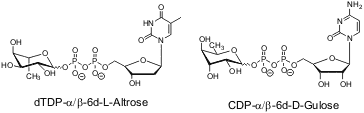
In previous work we have shown, that N-, S-nucleophiles and other O-nucleophiles also effected this initial reaction. Our target is the opening of the cycloSal phosphate triester by a phosphate monoester, which leads inevitably to the desired pyrophosphate. We choose two vantage points, on the one hand we will apply cycloSal-pyranose-1-phosphates (reaction with nucleotides) and on the other hand cycloSal-nucleotides (reaction with pyranose-1-phosphates). The target structures are the following nucleotide diphosphate pyranoses:
Contact:
|
Prof. Dr. Chris Meier Address: Institut für Organische Chemie Universität Hamburg Martin-Luther-King-Platz 6 20146 Hamburg Telefon: 040-42838-4324 Telefax: 040-42838-2495 E-Mail: chris.meier@chemie.uni-hamburg.de http://www.chemie.uni-hamburg.de/oc/meier/ |

|